Computational Modeling and Experimental Investigation of CO2-Hydrocarbon System Within Cross-Scale Porous Media
- PMID: 39860149
- PMCID: PMC11767899
- DOI: 10.3390/molecules30020277
Computational Modeling and Experimental Investigation of CO2-Hydrocarbon System Within Cross-Scale Porous Media
Abstract
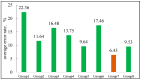
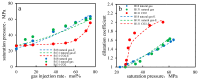
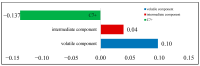
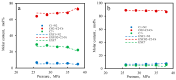
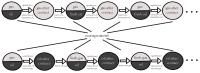
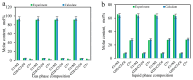
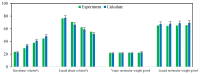
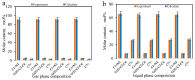
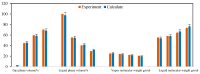
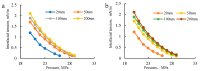
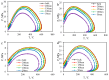
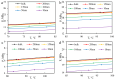
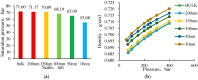
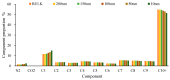
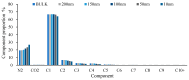
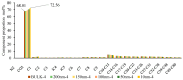
CO2 flooding plays a crucial role in enhancing oil recovery and achieving carbon reduction targets, particularly in unconventional reservoirs with complex pore structures. The phase behavior of CO2 and hydrocarbons at different scales significantly affects oil recovery efficiency, yet its underlying mechanisms remain insufficiently understood. This study improves existing thermodynamic models by introducing Helmholtz free energy as a convergence criterion and incorporating adsorption effects in micro- and nano-scale pores. This study refines existing thermodynamic models by incorporating Helmholtz free energy as a convergence criterion, offering a more accurate representation of confined phase behavior. Unlike conventional Gibbs free energy-based models, this approach effectively accounts for confinement-induced deviations in phase equilibrium, ensuring improved predictive accuracy for nanoscale reservoirs. Additionally, adsorption effects in micro- and nano-scale pores are explicitly integrated to enhance model reliability. A multi-scale thermodynamic model for CO2-hydrocarbon systems is developed and validated through physical simulations. Key findings indicate that as the scale decreases from bulk to 10 nm, the bubble point pressure shows a deviation of 5% to 23%, while the density of confined fluids increases by approximately 2%. The results also reveal that smaller pores restrict gas expansion, leading to an enhanced CO2 solubility effect and stronger phase mixing behavior. Through phase diagram analysis, density expansion, multi-stage contact, and differential separation simulations, we further clarify how confinement influences CO2 injection efficiency. These findings provide new insights into phase behavior changes in confined porous media, improving the accuracy of CO2 flooding predictions. The proposed model offers a more precise framework for evaluating phase transitions in unconventional reservoirs, aiding in the optimization of CO2-based enhanced oil recovery strategies.
Keywords: CO2 flooding; confined fluids; enhanced oil recovery; multi-scale; phase behavior.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



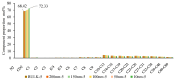
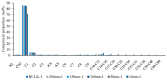
References
-
- Lau H.C., Ramakrishna S., Zhang K., Radhamani A.V. The Role of Carbon Capture and Storage in the Energy Transition. Energy Fuels. 2021;35:7364–7386. doi: 10.1021/acs.energyfuels.1c00032. - DOI
-
- Martin-Roberts E., Scott V., Flude S., Johnson G., Haszeldine R.S., Gilfillan S. Carbon capture and storage at the end of a lost decade. One Earth. 2021;4:1569–1584. doi: 10.1016/j.oneear.2021.10.002. - DOI
-
- Hoteit H. Proper Modeling of Diffusion in Fractured Reservoirs; Proceedings of the SPE Reservoir Simulation Symposium; The Woodlands, TX, USA. 21–23 February 2011.
-
- Erfan M., Badrul Mohamed J., Amin A., Hossein H., Nur Hidayati Binti O., Aqilah D., Siti Nurliyana Binti Che Mohamed H., Rozana Azrina Binti S. CO2-EOR/Sequestration: Current Trends and Future Horizons. In: Ariffin S., editor. Enhanced Oil Recovery Processes. IntechOpen; Rijeka, Croatia: 2019. Chapter 7.
-
- Song Y., Jun S., Na Y., Kim K., Jang Y., Wang J. Geomechanical challenges during geological CO2 storage: A review. Chem. Eng. J. 2023;456:140968. doi: 10.1016/j.cej.2022.140968. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

