Comparative Analysis of γ-Cyclodextrin, Perilla Oil, and Their Inclusion Complexes on Liver Injury and Dyslipidemia Associated with Elevated Gastrointestinal 12-Hydroxylated Bile Acid Levels
- PMID: 39860151
- PMCID: PMC11767548
- DOI: 10.3390/molecules30020281
Comparative Analysis of γ-Cyclodextrin, Perilla Oil, and Their Inclusion Complexes on Liver Injury and Dyslipidemia Associated with Elevated Gastrointestinal 12-Hydroxylated Bile Acid Levels
Abstract
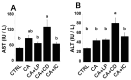
Our previous study demonstrated that γ-cyclodextrin (γ-CD)-perilla oil inclusion complexes increase plasma α-linolenic acid and eicosapentaenoic acid levels in healthy rats without adverse effects. The present study examined the effects of perilla oil, γ-CD, and their inclusion complexes on rats fed cholic acid (CA) to mimic the elevated gastrointestinal 12-hydroxylated (12OH) bile acid levels in high-fat diet-fed rats. Rats fed CA (CA group) tended to have higher AST, ALT, plasma total cholesterol (T-CHO), and triglyceride (TG) levels compared to controls fed a standard diet without CA. Rats fed CA and perilla oil (CA+LP group) showed a tendency for lower AST and plasma TG levels than those in the CA group. Rats fed CA and γ-CD (CA+CD group) had significantly higher AST, ALT, plasma T-CHO, and TG levels than the controls, indicating severe liver injury and dyslipidemia. Rats fed CA and the γ-CD-perilla oil inclusion complex (CA+IC group) had significantly lower AST and ALT levels than the CA+CD rats, with a trend towards lower plasma T-CHO and TG levels. Plasma α-linolenic acid and eicosapentaenoic acid levels were significantly higher in the CA+LP and CA+IC groups than in the controls and CA+CD groups. However, the CA+IC group tended to have lower α-linolenic acid levels and significantly lower eicosapentaenoic acid levels than the CA+LP group. This suggests an accelerated conversion of α-linolenic acid to eicosapentaenoic acid in the CA+IC group, which may contribute to the attenuation of liver injury and dyslipidemia. These findings suggest that γ-CD may exacerbate liver injury and dyslipidemia caused by elevated gastrointestinal 12OH bile acid levels, whereas γ-CD-perilla oil inclusion complexes may ameliorate these effects by altering fatty acid metabolism. Furthermore, we recommend evaluating γ-CD safety in both healthy and pathological models and carefully selecting compounds co-ingested with γ-CD.
Keywords: 12-hydroxylated bile acids; Perilla oil; cholic acid; dyslipidemia; eicosapentaenoic acid; fatty acid; inclusion complex; liver injury; α-linolenic acid; γ-cyclodextrin.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to the present study.
Figures
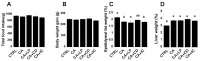
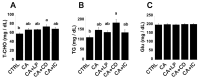
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

