Mechanistic Pathways in Cyanide-Mediated Benzoin Condensation: A Comprehensive Electron Localisation Function (ELF) and Catastrophe Theory Analysis of the Umpolung Reaction
- PMID: 39860246
- PMCID: PMC11767247
- DOI: 10.3390/molecules30020378
Mechanistic Pathways in Cyanide-Mediated Benzoin Condensation: A Comprehensive Electron Localisation Function (ELF) and Catastrophe Theory Analysis of the Umpolung Reaction
Abstract
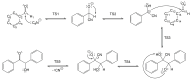
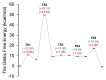
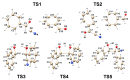
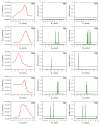
This research investigates the mechanism of the cyanide-type umpolung reaction in benzoin condensation using topological analysis of ELF and catastrophe theory. The study achieves a comprehensive understanding of the evolution of chemical bonds and non-bonding electron density in the reaction of benzaldehyde and cyanide ions. The results reveal that the reaction proceeds through five transition state structures, with the formation of Lapworth's cyanohydrin being the rate-determining step. The study characterises topological catastrophes in the evolution of the ELF field and provides a detailed description of the evolution of electron density in the mechanism of the reaction. An in-depth analysis of ELF catastrophes confirms the well-established Lapworth mechanism.
Keywords: BET; ELF; benzoin condensation; umpolung reaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Mechanism insight into the cyanide-catalyzed benzoin condensation: a density functional theory study.J Phys Chem A. 2010 Sep 2;114(34):9222-30. doi: 10.1021/jp103031q. J Phys Chem A. 2010. PMID: 20795738
-
The Mechanism of Boron-Carbon Bond Formation in the DA Reaction of the Pyridine Adduct of Borabenzene with Acetylene: A Topological Analysis of the ELF Function and Catastrophe Theory.Molecules. 2025 May 28;30(11):2357. doi: 10.3390/molecules30112357. Molecules. 2025. PMID: 40509242 Free PMC article.
-
Evolution of chemical bonding and electron density rearrangements during D(3h) → D(3d) reaction in monolayered TiS2: a QTAIM and ELF study.J Comput Chem. 2014 Aug 15;35(22):1641-5. doi: 10.1002/jcc.23662. Epub 2014 Jun 19. J Comput Chem. 2014. PMID: 24943852
-
Advances in chemoselective intermolecular cross-benzoin-type condensation reactions.Org Biomol Chem. 2017 Aug 23;15(33):6867-6887. doi: 10.1039/c7ob01662j. Org Biomol Chem. 2017. PMID: 28809427 Review.
-
A 30-Year Journey Towards an Accelerated Scheme for Visualizing ELF Basins in Molecules.J Comput Chem. 2025 Jun 15;46(16):e70146. doi: 10.1002/jcc.70146. J Comput Chem. 2025. PMID: 40485129 Review.
References
-
- Lapworth A. XCVI.—Reactions involving the addition of hydrogen cyanide to carbon compounds. J. Chem. Soc. Trans. 1903;83:995–1005. doi: 10.1039/CT9038300995. - DOI
LinkOut - more resources
Full Text Sources

