Peripheral Extracellular Vesicles for Diagnosis and Prognosis of Resectable Lung Cancer: The LUCEx Study Protocol
- PMID: 39860417
- PMCID: PMC11765880
- DOI: 10.3390/jcm14020411
Peripheral Extracellular Vesicles for Diagnosis and Prognosis of Resectable Lung Cancer: The LUCEx Study Protocol
Abstract
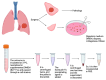
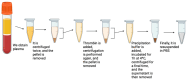
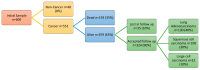
Background/Objectives: Lung cancer is the primary cause of cancer-related deaths. Most patients are typically diagnosed at advanced stages. Low-dose computed tomography (LDCT) has been proven to reduce lung cancer mortality, but screening programs using LDCT are associated with a high number of false positives and unnecessary thoracotomies. It is therefore imperative that a certain diagnosis is refined, especially in cases of solitary pulmonary nodules that are difficult to technically access for an accurate preoperative diagnosis. Extracellular vesicles (EVs) involved in intercellular communication may be an innovative biomarker for diagnosis and therapeutic strategies in lung cancer, regarding their ability to carry tumor-specific cargo. The aim of the LUCEx study is to determine if extracellular vesicle cargoes from both lung tissue and blood could provide complementary information to screen lung cancer patients and enable personalized follow-up after the surgery. Methods: The LUCEx study is a prospective study aiming to recruit 600 patients with lung cancer and 50 control subjects (false positives) undergoing surgery after diagnostic imaging for suspected pulmonary nodules using computed tomography (CT) scans. These patients will undergo curative surgery at the Department of Thoracic Surgery of the Miguel Servet Hospital in Zaragoza, Spain, and will be followed-up for at least 5 years. At baseline, samples from both tumor distal lung tissue and preoperative peripheral blood will be collected and processed to compare the quantity and content of EVs, particularly their micro-RNA (miRNA) cargo. At the third and fifth years of follow-up, CT scans, functional respiratory tests, and blood extractions will be performed. Discussion: Extracellular vesicles and their miRNA have emerged as promising tools for the diagnosis and prognosis of several diseases, including cancer. The LUCEx study, based on an observational clinical cohort, aims to understand the role of these vesicles and their translational potential as complementary tools for imaging diagnosis and prognosis.
Keywords: diagnosis; exosomes; extracellular vesicle; lung cancer; micro-RNA; prognosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The effect of direct referral for fast CT scan in early lung cancer detection in general practice. A clinical, cluster-randomised trial.Dan Med J. 2015 Mar;62(3):B5027. Dan Med J. 2015. PMID: 25748876 Clinical Trial.
-
Diagnostic work-up and surgery in participants of the Gdansk lung cancer screening programme: the incidence of surgery for non-malignant conditions.Interact Cardiovasc Thorac Surg. 2013 Dec;17(6):969-73. doi: 10.1093/icvts/ivt388. Epub 2013 Sep 5. Interact Cardiovasc Thorac Surg. 2013. PMID: 24008181 Free PMC article.
-
[Lung cancer screening in high-risk subjects: early detection with LDCT and risk stratification using miRNA-based blood test].Epidemiol Prev. 2016 Jan-Feb;40(1 Suppl 1):42-50. doi: 10.19191/EP16.1S1.P042.029. Epidemiol Prev. 2016. PMID: 26951732 Italian.
-
Screen-detected solid nodules: from detection of nodule to structured reporting.Transl Lung Cancer Res. 2021 May;10(5):2335-2346. doi: 10.21037/tlcr-20-296. Transl Lung Cancer Res. 2021. PMID: 34164281 Free PMC article. Review.
-
Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications.Cell Commun Signal. 2019 Jul 10;17(1):73. doi: 10.1186/s12964-019-0390-y. Cell Commun Signal. 2019. PMID: 31291956 Free PMC article. Review.
Cited by
-
Extracellular vesicle-associated epidermal growth factor receptor as a potential liquid biopsy biomarker in lung adenocarcinoma: a case-control study.J Yeungnam Med Sci. 2025;42:36. doi: 10.12701/jyms.2025.42.36. Epub 2025 May 23. J Yeungnam Med Sci. 2025. PMID: 40414258 Free PMC article.
References
-
- Ost D.E., Yeung S.C.J., Tanoue L.T., Gould M.K. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:121–141. doi: 10.1378/chest.12-2352. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources