Restoration of Genuine Sensation and Proprioception of Individual Fingers Following Transradial Amputation with Targeted Sensory Reinnervation as a Mechanoneural Interface
- PMID: 39860422
- PMCID: PMC11765609
- DOI: 10.3390/jcm14020417
Restoration of Genuine Sensation and Proprioception of Individual Fingers Following Transradial Amputation with Targeted Sensory Reinnervation as a Mechanoneural Interface
Abstract
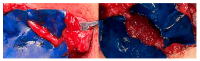
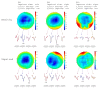
Background/Objectives: Tactile gnosis derives from the interplay between the hand's tactile input and the memory systems of the brain. It is the prerequisite for complex hand functions. Impaired sensation leads to profound disability. Various invasive and non-invasive sensory substitution strategies for providing feedback from prostheses have been unsuccessful when translated to clinical practice, since they fail to match the feeling to genuine sensation of the somatosensory cortex. Methods: Herein, we describe a novel surgical technique for upper-limb-targeted sensory reinnervation (ulTSR) and report how single digital nerves selectively reinnervate the forearm skin and restore the spatial sensory capacity of single digits of the amputated hand in a case series of seven patients. We explore the interplay of the redirected residual digital nerves and the interpretation of sensory perception after reinnervation of the forearm skin in the somatosensory cortex by evaluating sensory nerve action potentials (SNAPs), somatosensory evoked potentials (SEPs), and amputation-associated pain qualities. Results: Digital nerves were rerouted and reliably reinnervated the forearm skin after hand amputation, leading to somatotopy and limb maps of the thumb and four individual fingers. SNAPs were obtained from the donor digital nerves after stimulating the recipient sensory nerves of the forearm. Matching SEPs were obtained after electrocutaneous stimulation of the reinnervated skin areas of the forearm where the thumb, index, and little fingers are perceived. Pain incidence was significantly reduced or even fully resolved. Conclusions: We propose that ulTSR can lead to higher acceptance of prosthetic hands and substantially reduce the incidence of phantom limb and neuroma pain. In addition, the spatial restoration of lost-hand sensing and the somatotopic reinnervation of the forearm skin may serve as a machine interface, allowing for genuine sensation and embodiment of the prosthetic hand without the need for complex neural coding adjustments.
Keywords: electroencephalogram; hand amputation; limb map; neuroma prophylaxis; neuropathic pain; pattern recognition; phantom hand; phantom limb map; phantom limb pain; sensory feedback; targeted muscle reinnervation; targeted sensory reinnervation; transradial amputation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

