Clinical Outcomes Following a Switch of Therapy to Faricimab in Patients Affected by Neovascular Age-Related Macular Degeneration
- PMID: 39860429
- PMCID: PMC11766198
- DOI: 10.3390/jcm14020423
Clinical Outcomes Following a Switch of Therapy to Faricimab in Patients Affected by Neovascular Age-Related Macular Degeneration
Abstract
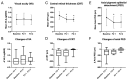
Objectives: In this study, we evaluated clinical outcomes following a therapy switch to Faricimab, in a patient cohort affected by neovascular age-related macular degeneration (nAMD), having received prior intravitreal anti-VEGF therapy. Methods: A retrospective investigation, including 28 eyes of 23 patients, treated for nAMD at the University Medical Center Mainz, Germany was performed. A switch in therapy to Faricimab was conducted, due to an inadequate response to the previous anti-VEGF treatment. Visual acuity (VA), central retinal thickness (CRT), and axial pigment epithelial detachment (PED) height were analyzed, following the first (FU 1) and second (FU 2) Faricimab injection series. Further, a subgroup analysis was conducted to compare Faricimab responders and diminished responders, as well as an exploratory data analyses to evaluate potential influencing factors on VA and CRT changes. Results: The mean age of patients was 82 years, with an average prior anti-VEGF treatment duration of 4.4 years and an average of 33 prior injections. Following Faricimab, at FU 1, significant reductions in CRT (from 335.8 µm to 260.0 µm, p < 0.01) and axial PED height (from 177 µm to 116 µm, p < 0.01) were observed. At FU 2, anatomical improvements were stable. No significant improvements in VA were observed, with LogMAR remaining stable at FU 1 and FU 2. In the subgroup comparison, eight eyes fulfilled the responder criteria, exhibiting morphological and functional improvements following intravitreal Faricimab. Further, a bigger baseline CRT correlated with a bigger post-treatment CRT and a longer prior treatment duration, and a worse baseline VA correlated with a worse post-Faricimab VA. No adverse events were noted following the switch to Faricimab. Conclusions: Following a switch to Faricimab, significant anatomical improvements were observed, while VA remained stable. Baseline CRT, prior treatment duration, and baseline LogMAR were associated with clinical outcomes post the switch to Faricimab. Further investigations into long-term outcomes are necessary to evaluate the sustained efficacy of Faricimab.
Keywords: AMD; Faricimab; Retina; anti-VEGF; macular degeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Korb C.A., Elbaz H., Schuster A.K., Nickels S., Ponto K.A., Schulz A., Wild P.S., Münzel T., Beutel M.E., Schmidtmann I., et al. Five-year cumulative incidence and progression of age-related macular degeneration: Results from the German population-based Gutenberg Health Study (GHS) Graefe’s Arch. Clin. Exp. Ophthalmol. 2022;260:55–64. doi: 10.1007/s00417-021-05312-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

