Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial
- PMID: 39860586
- PMCID: PMC11766411
- DOI: 10.3390/jcm14020581
Artificial Intelligence for Adenoma and Polyp Detection During Screening and Surveillance Colonoscopy: A Randomized-Controlled Trial
Abstract
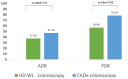
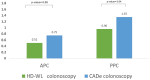
Background: Colorectal cancer (CRC) is the second leading cause of cancer death in Kuwait. The effectiveness of colonoscopy in preventing CRC is dependent on a high adenoma detection rate (ADR). Computer-aided detection can identify (CADe) and characterize polyps in real time and differentiate benign from neoplastic polyps, but its role remains unclear in screening colonoscopy. Methods: This was a randomized-controlled trial (RCT) enrolling patients 45 years of age or older presenting for outpatient screening or surveillance colonoscopy (Kuwait clinical trial registration number 2047/2022). Patients with a history of inflammatory bowel disease, alarm symptoms, familial polyposis syndrome, colon resection, or poor bowel preparation were excluded. Patients were randomly assigned to either high-definition white-light (HD-WL) colonoscopy (standard of care) or HD-WL colonoscopy with the CADe system. The primary outcome was ADR. The secondary outcomes included polyp detection rate (PDR), adenoma per colonoscopy (APC), polyp per colonoscopy (PPC), and accuracy of polyp characterization. Results: From 1 September 2022 to 1 March 2023, 102 patients were included and allocated to either the HD-WL colonoscopy group (n = 51) or CADe group (n = 51). The mean age was 52.8 years (SD 8.2), and males represented 50% of the cohort. Screening for CRC accounted for 94.1% of all examinations, while the remaining patients underwent surveillance colonoscopy. A total of 121 polyps were detected with an average size of 4.18 mm (SD 5.1), the majority being tubular adenomas with low-grade dysplasia (47.1%) and hyperplastic polyps (46.3%). There was no difference in the overall bowel preparation, insertion and withdrawal times, and adverse events between the two arms. ADR (primary outcome) was non-significantly higher in the CADe group compared to the HD colonoscopy group (47.1% vs. 37.3%, p = 0.3). Among the secondary outcomes, PDR (78.4% vs. 56.8%, p = 0.02) and PPC (1.35 vs. 0.96, p = 0.04) were significantly higher in the CADe group, but APC was not (0.75 vs. 0.51, p = 0.09). Accuracy in characterizing polyp histology was similar in both groups. Conclusions: In this RCT, the artificial intelligence system showed a non-significant trend towards improving ADR among Kuwaiti patients undergoing screening or surveillance colonoscopy compared to HD-WL colonoscopy alone, while it significantly improved the detection of diminutive polyps. A larger multicenter study is required to detect the true effect of CADe on the detection of adenomas.
Keywords: CADe; adenoma; colon cancer; colonoscopy; polyp.
Conflict of interest statement
Ali Alali, Ahmad Alhashmi, Nawal Alotaibi, Narges Ali, Maryam Alali, and Ahmad Alfadhli have no financial relationships relevant to this publication to disclose.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials