Eicosapentaenoic Acid and Docosahexaenoic Acid as an Antimicrobial Agent in Orthopedics-An In Vitro Study About the Race for Surface
- PMID: 39861018
- PMCID: PMC11768219
- DOI: 10.3390/pathogens14010057
Eicosapentaenoic Acid and Docosahexaenoic Acid as an Antimicrobial Agent in Orthopedics-An In Vitro Study About the Race for Surface
Abstract
Background: The burden of prosthetic joint infection in combination with antibiotic-resistant bacterial strains is a rising dilemma for patients experiencing total joint replacements. Around 0.8-2% of patients experience prosthetic joint infections, while up to 21% of patients are considered fatal cases after 5 years. Staphylococcus aureus is one of the main reasons for prosthetic joint infections. Its capability of forming biofilms and developing mechanisms against antibiotics is one of the most dangerous clinical topics being currently discussed. Previous studies have shown the promising results of omega-3 fatty acids as an antimicrobial agent against Staphylococcus aureus. Though an antimicrobial effect has been examined, the influence of polyunsaturated fatty acids on Staphylococcus aureus in the presence of human osteoblasts has not been reported yet. In this study, we aimed to investigate the influence of omega-3 fatty acids on the biofilm formation of Staphylococcus aureus ATCC 29213 in the presence of hFOB 1.19 cells. The co-culture setup helped to examine the influence of omega-3 fatty acids on the race for surface to simulate prosthetic joint infections.
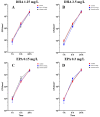
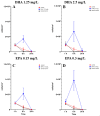
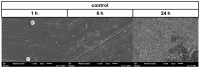
Methods: In this study, we tested Staphylococcus aureus ATCC 29213 co-cultured with human fetal osteoblasts hFOB 1.19 in the presence of sub-MIC and MIC concentrations of docosahexaenoic acid (1.25 mg/L, 2.5 mg/L) and eicosapentaenoic acid (0.15 mg/L, 0.3 mg/L) after 1, 6 and 24 h of incubation. After establishing the co-culture, cell culture and biofilm, we performed colony-forming unit counting and cell counting to examine cell survivability. In addition, we carried out scanning electron microscopy to study the race for surface behaviour of the cells.
Results: We found a protective influence of omega-3 fatty acids on osteoblasts when present in co-culture with Staphylococcus aureus after 6 h of incubation. Omega-3 fatty acids increase the cell survival of osteoblasts after 6 h in a co-culture with bacteria and are able to influence the race for surface. In this study, the strain of Staphylcoccus aureus ATCC 29213 showed signs of growth inhibition within the first 6 h.
Conclusions: Omega-3 fatty acids can be a valuable antimicrobial agent in terms of decreasing the risk of on-site infection during surgery. Omega-3 fatty acids were shown to decrease the bacterial load within the first 6 h of incubation and increase the survivability of osteoblasts.
Keywords: PJI; Staphylococcus aureus; biofilm; implant-related infections; omega-3 fatty acids; orthopedics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Jin X., Luxan B.G., Hanly M., Pratt N.L., Harris I., de Steiger R., Graves S.E., Jorm L. Estimating incidence rates of periprosthetic joint infection after hip and knee arthroplasty for osteoarthritis using linked registry and administrative health data. Bone Jt. J. 2022;104:1060–1066. doi: 10.1302/0301-620X.104B9.BJJ-2022-0116.R1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

