Metal-Coordinated Polymer-Inorganic Hybrids: Synthesis, Properties, and Application
- PMID: 39861209
- PMCID: PMC11768156
- DOI: 10.3390/polym17020136
Metal-Coordinated Polymer-Inorganic Hybrids: Synthesis, Properties, and Application
Abstract
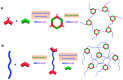
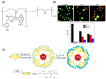
This review examines the recent advancements and unique properties of polymer-inorganic hybrid materials formed through coordination bonding (Class II hybrids), which enable enhanced functionality and stability across various applications. Here, we categorize these materials based on properties gained through complexation, focusing on electrical conductivity, thermal stability, photophysical characteristics, catalytic activity, and nanoscale self-assembly. Two major synthetic approaches to making these hybrids include homogeneous and heterogeneous methods, each with distinct tradeoffs: Homogeneous synthesis is straightforward but requires favorable mixing between inorganic and polymer species, which are predominantly water-soluble complexes. In contrast, heterogeneous methods are post-processing techniques that provide high area selectivity for inorganic precursors, allowing precise integration within polymer matrices. Finally, we highlight the role of hybrid linkers, namely metallosupramolecular polymers, in creating structural diversity. These can be organized into three main groups: metal-organic frameworks (MOFs), coordination polymers (CPs), and supramolecular coordination complexes (SCCs). Each of these groups introduces unique structural and functional properties that expand the potential applications of hybrid materials.
Keywords: Metal-Coordinated Polymers; Polymer-Inorganic Hybrids; Synthesis Approaches; block copolymers; coordination bonding; metallosupramolecular polymers.
Conflict of interest statement
The authors declare no competing financial interests.
Figures








References
-
- Wiesner U., Matyjaszewski K., Möller M. Polymer Science: A Comprehensive Reference. Elsevier; Amsterdam, The Netherlands: 2012. Hybrid Polymer–Inorganic Nanostructures; pp. 129–140.
-
- Katagiri K., Ariga K., Kikuchi J. Novel class of organic-inorganic hybrid vesicle “Cerasome” derived from various amphiphiles with alkoxysilyl head. Stud. Surf. Sci. Catal. 2001;132:599–602. doi: 10.1016/S0167-2991(01)82162-5. - DOI
-
- Judeinstein P., Sanchez C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996;6:511–525. doi: 10.1039/JM9960600511. - DOI
-
- Mir S., Nagahara L., Thundat T., Mokarian-Tabari P., Furukawa H., Khosla A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018;165:B3137–B3156. doi: 10.1149/2.0191808jes. - DOI
-
- Livage J., Henry M., Sanchez C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988;18:259–341. doi: 10.1016/0079-6786(88)90005-2. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

