Acylase-Based Coatings on Sandblasted Polydimethylsiloxane-Based Materials for Antimicrobial Applications
- PMID: 39861255
- PMCID: PMC11768103
- DOI: 10.3390/polym17020182
Acylase-Based Coatings on Sandblasted Polydimethylsiloxane-Based Materials for Antimicrobial Applications
Abstract
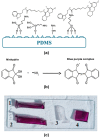
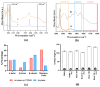
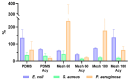
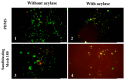
Indwelling medical devices, such as urinary catheters, often experience bacterial colonization, forming biofilms that resist antibiotics and the host's immune defenses through quorum sensing (QS), a chemical communication system. This study explores the development of antimicrobial coatings by immobilizing acylase, a quorum-quenching enzyme, on sandblasted polydimethylsiloxane (PDMS) surfaces. PDMS, commonly used in medical devices, was sandblasted to increase its surface roughness, enhancing acylase attachment. FTIR analysis confirmed that acylase retained its three-dimensional structure upon immobilization, preserving its enzymatic activity. The antibacterial efficacy of the coatings was tested against Pseudomonas aeruginosa (P. aeruginosa) (a common biofilm-forming pathogen), Staphylococcus aureus (S. aureus), and Escherichia coli (E. coli). The results showed that sandblasted PDMS surfaces had improved bacterial adhesion due to increased focal adhesion points, but acylase-functionalized surfaces had significantly reduced bacterial attachment and biofilm formation. Notably, the coatings inhibited P. aeruginosa growth by 40% under static conditions, demonstrating the potential of acylase-functionalized PDMS for medical applications. This approach offers a promising strategy for creating antimicrobial surfaces that prevent biofilm-related infections in urinary catheters and other medical devices. The findings highlight the dual role of surface roughness in enhancing enzyme attachment while reducing bacterial adhesion through effective QS inhibition.
Keywords: PDMS; acylase; quorum quenching; quorum sensing; sandblasting.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Casini M. Chapter 7—Advanced construction materials. In: Casini M., editor. Construction 4.0. Woodhead Publishing; Sawston, UK: 2022. pp. 337–404. (Woodhead Publishing Series in Civil and Structural Engineering). - DOI
-
- Contreras-Ramos M., Mansell T.J. Leveraging quorum sensing to manipulate microbial dynamics. Curr. Opin. Biomed. Eng. 2021;19:100306. doi: 10.1016/j.cobme.2021.100306. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

