Effects of Hydroxyapatite Additions on Alginate Gelation Kinetics During Cross-Linking
- PMID: 39861314
- PMCID: PMC11768125
- DOI: 10.3390/polym17020242
Effects of Hydroxyapatite Additions on Alginate Gelation Kinetics During Cross-Linking
Abstract
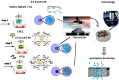
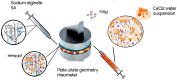
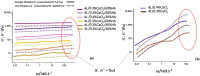
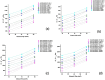
Alginate hydrogels have gathered significant attention in biomedical engineering due to their remarkable biocompatibility, biodegradability, and ability to encapsulate cells and bioactive molecules, but much less has been reported on the kinetics of gelation. Scarce experimental data are available on cross-linked alginates (AL) with bioactive components. The present study addressed a novel method for defining the crosslinking mechanism using rheological measurements for aqueous mixtures of AL and calcium chloride (CaCl2) with the presence of hydroxyapatite (HAp) as filler particles. The time-dependent crosslinking behaviour of these mixtures was exploited using a plate-plate rheometer, when crosslinking occurs due to calcium ions (Ca2+) binding to the guluronic acid blocks within the AL polymer, forming a stable "egg-box" structure. To reveal the influence of HAp particles as filler on crosslinked sample morphology, after rheological measurement and crosslinking, crosslinked samples were freeze-dried and their morphology was assessed using an optical microscope and SEM. It was found that the addition of HAp particles, which are known to enhance the mechanical properties and biocompatibility of crosslinked AL gels, significantly decreased (usually rapidly) the interaction between the Ca2+ and AL chains. In this research, the physical "shielding" effect of HAp particles on the crosslinking of AL with Ca2+ ions has been observed for the first time, and its crosslinking behaviour was defined using rheological methods. After crosslinking and rheometer measurements, the samples were further evaluated for morphological properties and the observations were correlated with their dewatering properties. While the presence of HAp particles led to a slower crosslinking process and a more uniform development of the rheological parameters, it also led to a more uniform porosity and improved dewatering properties. The observed effects allow for a better understanding of the crosslinking process kinetics, which directly affects the physical and chemical properties of the AL gels. The shielding behaviour (retardation) of filler particles occurs when they physically or chemically block certain components in a mixture, delaying their interaction with other reactants. In hydrogel formulations, filler particles like hydroxyapatite (HAp) can act as barriers, adsorbing onto reactive components or creating physical separation, which slows the reaction rate and allows for controlled gelation or delayed crosslinking. This delayed reactivity is beneficial for precise control over the reaction timing, enabling the better manipulation of material properties such as crosslinking distribution, pore structure, and mechanical stability. In this research, the physical shielding effect of HAp particles was observed through changes in rheological properties during crosslinking and was dependent on the HAp concentration. The addition of HAp also enabled more uniform porosity and improved dewatering properties. The observed effects allow for a better understanding of the crosslinking process kinetics, which directly affects the physical and chemical properties of the AL gels.
Keywords: calcium alginate hydrogels; crosslinking kinetics; hydroxyapatite; rheology.
Conflict of interest statement
Author Monir Imani was employed by the company Mirka. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Maintaining dimensions and mechanical properties of ionically crosslinked alginate hydrogel scaffolds in vitro.J Biomed Mater Res A. 2008 Mar 15;84(4):899-907. doi: 10.1002/jbm.a.31375. J Biomed Mater Res A. 2008. PMID: 17647237
-
Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties.Biomaterials. 2001 Mar;22(6):511-21. doi: 10.1016/s0142-9612(00)00201-5. Biomaterials. 2001. PMID: 11219714
-
Influence of oligoguluronates on alginate gelation, kinetics, and polymer organization.Biomacromolecules. 2007 Aug;8(8):2388-97. doi: 10.1021/bm070208d. Epub 2007 Jun 28. Biomacromolecules. 2007. PMID: 17602585
-
Progress in Research on Metal Ion Crosslinking Alginate-Based Gels.Gels. 2024 Dec 27;11(1):16. doi: 10.3390/gels11010016. Gels. 2024. PMID: 39851986 Free PMC article. Review.
-
Hydroxyapatite-alginate Based Matrices for Drug Delivery.Curr Pharm Des. 2019;25(31):3406-3416. doi: 10.2174/1381612825666190906164003. Curr Pharm Des. 2019. PMID: 31490744 Review.
Cited by
-
Complex Networks in Phase-Separating Gels: A Computer Simulation Study.Polymers (Basel). 2025 Mar 25;17(7):880. doi: 10.3390/polym17070880. Polymers (Basel). 2025. PMID: 40219270 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

