Potential Effect of Cinnamaldehyde on Insulin Resistance Is Mediated by Glucose and Lipid Homeostasis
- PMID: 39861427
- PMCID: PMC11767522
- DOI: 10.3390/nu17020297
Potential Effect of Cinnamaldehyde on Insulin Resistance Is Mediated by Glucose and Lipid Homeostasis
Abstract
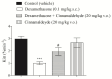
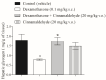
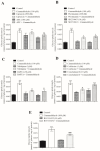
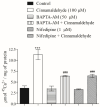
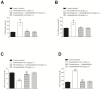
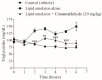
Diabetes mellitus is a metabolic syndrome that has grown globally to become a significant public health challenge. Hypothesizing that the plasma membrane protein, transient receptor potential ankyrin-1, is a pivotal target in insulin resistance, we investigated the mechanism of action of cinnamaldehyde (CIN), an electrophilic TRPA1 agonist, in skeletal muscle, a primary insulin target. Specifically, we evaluated the effect of CIN on insulin resistance, hepatic glycogen accumulation and muscle and adipose tissue glucose uptake. Furthermore, the in vitro role of CIN in glucose uptake and intracellular signaling was determined in insulin-resistant rats whose calcium influx was analyzed. Moreover, the serum lipid profile was assessed following short-term CIN treatment in rats, and lipid tolerance was analyzed. The effects of CIN on insulin resistance were mediated by TRPA1, with downstream signaling involving the activation of PI3-K, MAPK, PKC, as well as extracellular calcium and calcium release from intracellular stores. Additionally, cytoskeleton integrity was required for the complete action of CIN on glucose uptake in muscle. CIN also ameliorated the serum lipid profile and improved triglyceride tolerance following acute vivo exposure.
Keywords: adipose tissue; cinnamaldehyde; glucose uptake; glycogen; insulin resistant; lipids; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction.Naunyn Schmiedebergs Arch Pharmacol. 2019 Feb;392(2):243-258. doi: 10.1007/s00210-018-1583-4. Epub 2018 Nov 20. Naunyn Schmiedebergs Arch Pharmacol. 2019. PMID: 30460386
-
Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance.Dan Med J. 2014 Jul;61(7):B4890. Dan Med J. 2014. PMID: 25123125 Review.
-
5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice.Diabetologia. 2002 Jan;45(1):56-65. doi: 10.1007/s125-002-8245-8. Diabetologia. 2002. PMID: 11845224
-
Electrophilic Agonists Modulate the Transient Receptor Potential Ankyrin-1 Channels Mediated by Insulin and Glucagon-like Peptide-1 Secretion for Glucose Homeostasis.Pharmaceuticals (Basel). 2023 Aug 16;16(8):1167. doi: 10.3390/ph16081167. Pharmaceuticals (Basel). 2023. PMID: 37631083 Free PMC article.
-
Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety.Pharmacol Res. 2017 Aug;122:78-89. doi: 10.1016/j.phrs.2017.05.019. Epub 2017 May 27. Pharmacol Res. 2017. PMID: 28559210 Review.
References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
-
- Sodhi R.K., Kumar H., Singh R., Bansal Y., Singh Y., Kondepudi K.K., Bishnoi M., Kuhad A. Allyl isothiocyanate, a TRPA1 agonist, protects against olanzapine-induced hypothalamic and hepatic metabolic aberrations in female mice. Biochem. Pharmacol. 2024;222:116074. doi: 10.1016/j.bcp.2024.116074. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

