On the Biosynthesis of Bioactive Tryptamines in Black Cohosh (Actaea racemosa L.)
- PMID: 39861645
- PMCID: PMC11768127
- DOI: 10.3390/plants14020292
On the Biosynthesis of Bioactive Tryptamines in Black Cohosh (Actaea racemosa L.)
Abstract
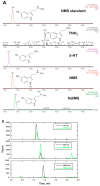
Botanical dietary supplements are widely used, but issues of authenticity, consistency, safety, and efficacy that complicate their poorly understood mechanism of action have prompted questions and concerns in the popular and scientific literature. Black cohosh (Actaea racemosa L., syn. Cimicifuga racemosa, Nutt., Ranunculaceae) is a multicomponent botanical therapeutic used as a popular remedy for menopause and dysmenorrhea and explored as a treatment in breast and prostate cancer. However, its use and safety are controversial. A. racemosa tissues contain the bioactive serotonin analog N-methylserotonin, which is thought to contribute to the serotonergic activities of black cohosh-containing preparations. A. racemosa has several TDC-like genes hypothesized to encode tryptophan decarboxylases (TDCs) converting L-tryptophan to tryptamine, a direct serotonin precursor in plants. Expression of black cohosh TDC1, TDC2, and TDC3 in Saccharomyces cerevisiae resulted in the production of tryptamine. TDC1 and TDC3 had approximately fourfold higher activity than TDC2, which was attributable to a variable Cys/Ser active site residue identified by site-directed mutagenesis. Co-expression in yeast of the high-activity black cohosh TDCs with the next enzyme in serotonin biosynthesis, tryptamine 5-hydroxylase (T5H), from rice (Oryza sativa) resulted in the production of serotonin, whereas co-expression with low-activity TDCs did not, suggesting that TDC activity is a rate-limiting step in serotonin biosynthesis. Two T5H-like sequences were identified in A. racemose, but their co-expression with the high-activity TDCs in yeast did not result in serotonin production. TDC expression was detected in several black cohosh tissues, and phytochemical analysis using LC-MS revealed several new tryptamines, including tryptamine and serotonin, along with N-methylserotonin and, interestingly, N-N-dimethyl-5-hydroxytryptamine (bufotenine), which may contribute to hepatotoxicity. Incubation of A. racemosa leaves with tryptamine and N-methyltryptamine resulted in increased concentrations of serotonin and N-methylserotonin, respectively, suggesting that methylation of tryptamine precedes hydroxylation in the biosynthesis of N-methylserotonin. This work indicates a significantly greater variety of serotonin derivatives in A. racemosa than previously reported. Moreover, the activities of the TDCs underscore their key role in the production of serotonergic compounds in A. racemosa. Finally, it is proposed that tryptamine is first methylated and then hydroxylated to form the black cohosh signature compound N-methylserotonin.
Keywords: Actaea racemosa; Cimicifuga racemosa; Rannunculaceae; black cohosh; secondary metabolism; serotonin; tryptamines; tryptophan decarboxylase.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Ma C., Kavalier A.R., Jiang B., Kennelly E.J. Metabolic profiling of Actaea species extracts using high performance liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A. 2011;1218:1461–1476. doi: 10.1016/j.chroma.2011.01.033. - DOI - PMC - PubMed
-
- McCoy J.A., Davis J.M., Camper N.D., Khan I., Bharathi A. Influence of rhizome propagule size on yields and triterpene glycoside concentrations of black cohosh [Actaea racemosa L. syn Cimicifuga racemosa (L.) Nuttal] HortScience. 2007;42:61–64. doi: 10.21273/HORTSCI.42.1.61. - DOI
-
- Predny M.L., DeAngelis P., Chamberlain J.L. Black Cohosh: An Annotated Bibliography. Southern Research Station; Asheville, NC, USA: 2006. pp. 1–108.
-
- Small C.J., Chamberlain J.L., Mathews D.S. Recovery of black cohosh (Actaea racemosa L.) following experimental harvests. Am. Midl. Nat. 2011;166:339–348. doi: 10.1674/0003-0031-166.2.339. - DOI
-
- Castelo-Branco C., Gambacciani M., Cano A., Minkin M.J., Rachoń D., Ruan X., Beer A.M., Schnitker J., Henneicke-von Zepelin H.H., Pickartz S. Review & meta-analysis: Isopropanolic black cohosh extract iCR for menopausal symptoms—An update on the evidence. Climacteric. 2021;24:109–119. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

