Sulforaphane Wrapped in Self-Assembled Nanomicelle Enhances the Effect of Sonodynamic Therapy on Glioma
- PMID: 39861683
- PMCID: PMC11769538
- DOI: 10.3390/pharmaceutics17010034
Sulforaphane Wrapped in Self-Assembled Nanomicelle Enhances the Effect of Sonodynamic Therapy on Glioma
Abstract
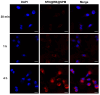
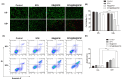
Background/Objectives: The two obstacles for treating glioma are the skull and the blood brain-barrier (BBB), the first of which forms a physical shield that increases the difficulties of traditional surgery or radiotherapy, while the latter prevents antitumor drugs reaching tumor sites. To conquer these issues, we take advantage of the high penetrating ability of sonodynamic therapy (SDT), combined with a novel nanocomplex that can easily pass the BBB. Methods: Through ultrasonic polymerization, the amphiphilic peptides (C18GR7RGDS) were self-assembled as a spherical shell encapsulating a sonosensitizer Rose Bengal (RB) and a plant-derived compound, sulforaphane (SFN), to form the nanocomplex SFN@RB@SPM. Results/Conclusions: SFN@RB@SPM can be internalized by the glioma cells through the tumor-targeting motif RGDS (abbreviated for the peptide sequence composed of arginine, glycine, aspartic acid, and serine), and further executes antitumor function during SDT. Also, SFN@RB@SPM could be easily taken up by U87-MG cells and cross the BBB in glioma-bearing mice during SDT. The mechanism investigation revealed that, compared with the SFN-free nanocomplex (RB@SPM), SFN@RB@SPM induced much more apoptosis of U87-MG cells in an ROS-dependent manner through the depletion of glutathione by SFN and the cavitation effect by SDT. In animal experiments, besides a significant reduction in tumor volume and a delay in losing body weight, H&E staining showed a massive infiltration of neutrophils adjacent to the tumor sites, indicating this novel nanocomplex SFN@RB@SPM can synergistically augment SDT efficacy, partially by enhancing the antitumor function of innate immunity.
Keywords: glioma; neutrophil infiltration; self-assembled nanomicelle; sonodynamic therapy; sulforaphane.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Bucci M.K., Maity A., Janss A.J., Belasco J.B., Fisher M.J., Tochner Z.A., Rorke L., Sutton L.N., Phillips P.C., Shu H.K. Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem, malignant gliomas: Results from a single center in the magnetic resonance imaging era. Cancer. 2004;101:817–824. doi: 10.1002/cncr.20422. - DOI - PubMed
Grants and funding
- 2021A-012-G/Young Innovative Talent Project of YongJiang Talent Introduction Programme from Ningbo Municipal Government
- 2023RC004/Bei An Talent Programme from Jiangbei District of Ningbo
- 2021J321/Ningbo Science and Technology Bureau
- 2021J328/Ningbo Science and Technology Bureau
- 2023J365/Ningbo Science and Technology Bureau
- 2022S030/Ningbo Science and Technology Bureau
- LQ24H160003/Zhejiang Provincial Natural Science Foundation
- 2023KY299/Medical Scientific Research Foundation of Zhejiang Province
- 2020KY848/Medical Scientific Research Foundation of Zhejiang Province
- 2024KY351/Medical Scientific Research Foundation of Zhejiang Province
- 2021YJY1006/Special Funding for Microfluidic Chip of Biomedicine of Ningbo Institute of Life and Health Industry, University of Chinese Academy of Sciences
- 2021YJY1005/Special Funding for Microfluidic Chip of Biomedicine of Ningbo Institute of Life and Health In-dustry, University of Chinese Academy of Sciences
- 2023HMYQ03/Ningbo No. 2 Hospital
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

