Improving Individualized Salbutamol Treatment: A Population Pharmacokinetic Model for Oral Salbutamol in Virtual Patients
- PMID: 39861686
- PMCID: PMC11768577
- DOI: 10.3390/pharmaceutics17010039
Improving Individualized Salbutamol Treatment: A Population Pharmacokinetic Model for Oral Salbutamol in Virtual Patients
Abstract
Background: Salbutamol, a short-acting β2-agonist used in asthma treatment, is available in multiple formulations, including inhalers, nebulizers, oral tablets, and intravenous, intramuscular, and subcutaneous routes. Each formulation exhibits distinct pharmacokinetic (PK) and pharmacodynamic (PD) profiles, influencing therapeutic outcomes and adverse effects. Although asthma management predominantly relies on inhaled salbutamol, understanding how these formulations interact with patient-specific characteristics could improve personalized medicine approaches, potentially uncovering the therapeutic benefits of alternative formulations for an individual patient. Herein, this study aims to analyze how covariates-such as age, weight, gender, body surface area (BSA), cytochrome P450 (CYP) expression, race, and health status-affect the therapeutic regime of orally administered salbutamol using population PK (popPK) modeling. The final model is intended as a tool to support the selection of optimal formulation and dosage regimen based on individual patient profiles.
Methods: A dataset of 40 virtual patients derived from a physiologically based PK (PBPK) model of oral salbutamol was included in the popPK model.
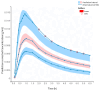
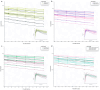
Results: A two-compartment model with first-order elimination and absorption, with a transit compartment, best described the plasma concentration-time profile following a 4 mg dose. Relationships were identified between gender and mean transit time (Mtt) and clearance (Cl), as well as the effects of weight and BSA on the volume of distribution of the central compartment (V1) and Cl, and a significant impact of health status on Cl.
Conclusions: Despite current contraindications for oral salbutamol, our findings suggest that certain individuals, particularly children, may benefit from oral treatment over inhalation. We also identified individual characteristics associated with increased salbutamol toxicity risk, indicating the need for dose adjustment or alternative administration. This study further highlights the potential of popPK modeling in personalized therapy through a fully in silico approach.
Keywords: PBPK modeling; covariates; interindividual variability; personalized medicine; pharmacokinetics; popPK modeling; salbutamol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Wang Z., Li Y., Gao Y., Fu Y., Lin J., Lei X., Zheng J., Jiang M. Global, Regional, and National Burden of Asthma and Its Attributable Risk Factors from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Respir. Res. 2023;24:169. doi: 10.1186/s12931-023-02475-6. - DOI - PMC - PubMed
-
- Craig S., Tuszynski M., Armstrong D. It Is Time to Stop Prescribing Oral Salbutamol. Aust. Fam. Physician. 2016;45:245–247. - PubMed
LinkOut - more resources
Full Text Sources

