Hydrogels of Poly(2-hydroxyethyl methacrylate) and Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Dermal Delivery Systems for Dexamethasone
- PMID: 39861710
- PMCID: PMC11768119
- DOI: 10.3390/pharmaceutics17010062
Hydrogels of Poly(2-hydroxyethyl methacrylate) and Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Dermal Delivery Systems for Dexamethasone
Abstract
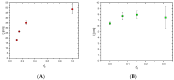
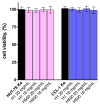
Background/Objectives: This study is an attempt to reveal the potential of two types of interpenetrating polymer network (IPN) hydrogels based on poly(2-hydroxyethyl methacrylate) (PHEMA) and poly(N,N-dimethylacrylamide) (PDMAM). These IPNs were evaluated for their potential for dermal delivery of the hydrophobic drug dexamethasone (DEX). Methods: The two types of IPNs were analyzed for their rheological behavior, swelling characteristics, and drug-loading capacity with DEX. Drug release profiles were studied in Franz diffusion cells in PBS media. Finally, the cytotoxicity of the PHEMA/PDMAM-based IPNs was studied against T-cell lymphoma cells (HUT-78) and a normal murine fibroblast cell line (CCL-1). Results: The rheological properties of these hydrogels show suitable mechanical properties for dermal application, with G' values of ~10 kPa. From the rheological data, the mesh size of these hydrogels was found to be influenced by the type of the IPN and its composition, varying between 6.5 and 50 nm. The loading capacity of both IPN types and DEX entrapment efficiency were highly influenced by the IPN's composition. The loading capacity of the IPNs can reach ~3.5%, with a DEX entrapment efficiency of ~35%. The PHEMA/PDMAM IPNs demonstrate an extended release profile with up to ~95% DEX released in 24 h, while PDMAM/PHEMA IPNs release no more than ~25% DEX in 24 h. The drug release profiles follow either non-Fickian diffusion (n~0.6) or case-II transport (n~0.9-1), depending on the IPN's composition. The PHEMA/PDMAM-based materials were found to be non-cytotoxic against HUT-78 and CCL-1 cells. Conclusions: The study reveals that the IPNs of PHEMA and PDMAM appear to be suitable platforms for dermal delivery of dexamethasone as they have appropriate mechanical properties, providing tools to control drug loading and release, and they are biocompatible with human skin cells.
Keywords: CCL-1; HUT-78; dermal application; dexamethasone; drug delivery; hydrogels; interpenetrating polymer networks; non-cytotoxic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride.Gels. 2025 Mar 25;11(4):240. doi: 10.3390/gels11040240. Gels. 2025. PMID: 40277676 Free PMC article.
-
Interpenetrating Polymer Networks of Poly(2-hydroxyethyl methacrylate) and Poly(N, N-dimethylacrylamide) as Potential Systems for Dermal Delivery of Dexamethasone Phosphate.Pharmaceutics. 2023 Sep 15;15(9):2328. doi: 10.3390/pharmaceutics15092328. Pharmaceutics. 2023. PMID: 37765296 Free PMC article.
-
Polyacrylamide/poly(2-(dimethylamino) Ethyl Methacrylate) Interpenetrating Polymer Networks as Drug Delivery Systems for Diclofenac Sodium.Gels. 2022 Nov 29;8(12):780. doi: 10.3390/gels8120780. Gels. 2022. PMID: 36547305 Free PMC article.
-
Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering.Adv Drug Deliv Rev. 2013 Aug;65(9):1172-87. doi: 10.1016/j.addr.2013.04.002. Epub 2013 Apr 17. Adv Drug Deliv Rev. 2013. PMID: 23603210 Review.
-
Controlled release of therapeutics using interpenetrating polymeric networks.Expert Opin Drug Deliv. 2015 Apr;12(4):669-88. doi: 10.1517/17425247.2014.974871. Epub 2014 Oct 24. Expert Opin Drug Deliv. 2015. PMID: 25341410 Review.
Cited by
-
Stimuli-Responsive Hydrogels of Poly(Methacrylic Acid)/Poly(N,N-dimethylacrylamide) Interpenetrating Polymer Networks as Drug Delivery Systems for Promethazine Hydrochloride.Gels. 2025 Mar 25;11(4):240. doi: 10.3390/gels11040240. Gels. 2025. PMID: 40277676 Free PMC article.
References
-
- Stanciu L., Diaz-Amaya S. Composite Biomaterials. Elsevier; Amsterdam, The Netherlands: 2022. pp. 149–169. Elsevier Ebooks. - DOI
-
- Jenkins A.D., Kratochvíl P., Stepto R.F.T., Suter U.W. Glossary of Basic Terms in Polymer Science (IUPAC Recommendations 1996) Pure Appl. Chem. 1996;68:2287–2311. doi: 10.1351/pac199668122287. - DOI
-
- Simeonov M., Kostova B., Vassileva E. Interpenetrating Polymer Networks of Poly(Acrylic Acid) and Polyacrylamide for Sustained Verapamil Hydrochloride Release. Macromol. Symp. 2015;358:225–231. doi: 10.1002/masy.201500014. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

