Retinal Protection of New Nutraceutical Formulation
- PMID: 39861721
- PMCID: PMC11769253
- DOI: 10.3390/pharmaceutics17010073
Retinal Protection of New Nutraceutical Formulation
Abstract
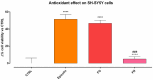
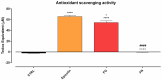
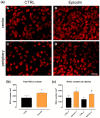
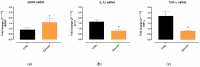
Background/Objectives: Retinal ganglion cell (RGC) protection represents an unmet need in glaucoma. This study assessed the neuroprotective, antioxidant, and anti-inflammatory effect of a new nutraceutical formulation named Epicolin, based on citicoline, homotaurine, epigallocatechin-3-gallate, forskolin, and vitamins, through in vitro and in vivo studies. Methods: The neuroprotective effect of Epicolin or its single components, and Epicolin compared to an untreated control and two marketed formulations [Formulation G (FG) and N (FN)], was evaluated in neuroblastoma cells (SH-SY5Y) challenged with staurosporine. The antioxidant potential and the scavenging activity of Epicolin compared to the untreated control, and FG and FN, was evaluated in SH-SY5Y cells and through oxygen radical absorbance capacity acellular assay, respectively. Moreover, the protective effect against hypoxic damage was evaluated in Muller cells (MIO-M1) subjected to hypoxia. The efficacy of Epicolin was also evaluated in DBA/2J glaucomatous mice through the use of a pattern electroretinogram (PERG), immunostaining, and real-time PCR. Results: Among the nutraceutical formulations tested, only Epicolin showed a significant neuroprotective effect on SH-SY5Y attributable to the synergistic action of its single ingredients. As for antioxidant and scavenging activity, Epicolin showed a higher efficacy compared to FG and FN. Furthermore, Epicolin showed the same protective effect on MIO-M1 cells reducing HIF-1α expression. Finally, Epicolin treatment on DBA/2J mice protected the RGCs from loss of function, as demonstrated by PERG analysis, and attenuated their death by enhancing brain-derived neurotrophic factor (BDNF) and reducing interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) expression. Conclusions: Epicolin, due to its neuroprotective, antioxidant, and anti-inflammatory properties, represents a promising potential treatment for glaucoma.
Keywords: RGC degeneration; citicoline; dietary supplement; epigallocatechin-3-gallate; forskolin; glaucoma; homotaurine; neuroprotection; retinal function.
Conflict of interest statement
The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. L.R.L.R., V.P. and C.Z. are coinventors of the international patent n. EP4356906A8·2024-07-03. L.R.L.R., V.P., S.V., G.D.P., M.C.C. and C.Z. are all employees at SIFI S.p.A. F.L., G.L.R., F.C., E.G. and C.B. declare no conflicts of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

