Exploration of the Role of Cyclophilins in Established Hepatitis B and C Infections
- PMID: 39861799
- PMCID: PMC11768883
- DOI: 10.3390/v17010011
Exploration of the Role of Cyclophilins in Established Hepatitis B and C Infections
Abstract
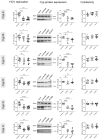
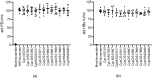
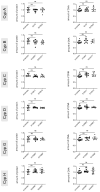
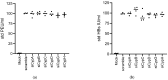
Cyclophilin (Cyp) inhibitors are of clinical interest in respect to their antiviral activities in the context of many viral infections including chronic hepatitis B and C. Cyps are a group of enzymes with peptidyl-prolyl isomerase activity (PPIase), known to be required for replication of diverse viruses including hepatitis B and C viruses (HBV and HCV). Amongst the Cyp family, the molecular mechanisms underlying the antiviral effects of CypA have been investigated in detail, but potential roles of other Cyps are less well studied in the context of viral hepatitis. Furthermore, most studies investigating the role of Cyps in viral hepatitis did not investigate the potential therapeutic effects of their inhibition in already-established infections but have rather been performed in the context of neo-infections. Here, we investigated the effects of genetically silencing Cyps on persistent HCV and HBV infections. We confirm antiviral effects of CypA and CypD knock down and demonstrate novel roles for CypG and CypH in HCV replication. We show, furthermore, that CypA silencing has a modest but reproducible impact on persistent HBV infections in cultured human hepatocytes.
Keywords: anti-viral treatment; cyclophilin; hepatitis virus; liver.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

