Development of a Luciferase Immunosorbent Assay for Detecting Crimean-Congo Hemorrhagic Fever Virus IgG Antibodies Based on Nucleoprotein
- PMID: 39861821
- PMCID: PMC11769549
- DOI: 10.3390/v17010032
Development of a Luciferase Immunosorbent Assay for Detecting Crimean-Congo Hemorrhagic Fever Virus IgG Antibodies Based on Nucleoprotein
Abstract
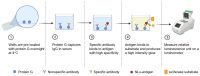
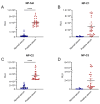
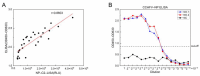
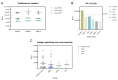
Crimean-Congo hemorrhagic fever (CCHF) is a serious tick-borne disease with a wide geographical distribution. Classified as a level 4 biosecurity risk pathogen, CCHF can be transmitted cross-species due to its aerosol infectivity and ability to cause severe hemorrhagic fever outbreaks with high morbidity and mortality. However, current methods for detecting anti-CCHFV antibodies are limited. This study aimed to develop a novel luciferase immunosorbent assay (LISA) for the detection of CCHFV-specific IgG antibodies. We designed specific antigenic fragments of the nucleoprotein and evaluated their sensitivity and specificity in detecting IgG in serum samples from mice and horses. In addition, we compared the efficacy of our LISA to a commercial enzyme-linked immunosorbent assay (ELISA). Our results demonstrated that the optimal antigen for detecting anti-CCHFV IgG was located within the stalk cut-off domain of the nucleoprotein. The LISA exhibited high specificity for serum samples from indicated species and significantly higher sensitivity (at least 128 times) compared with the commercial ELISA. The proposed CCHFV-LISA has the potential to facilitate serological diagnosis and epidemiological investigation of CCHFV in natural foci, providing valuable technical support for surveillance and early warning of this disease.
Keywords: Crimean–Congo hemorrhagic fever virus (CCHFV); IgG; detection; luciferase immunosorbent assay (LISA); nucleoprotein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
First report of Crimean-Congo hemorrhagic fever virus exposure in human and livestock populations, Center Region, Cameroon.Front Cell Infect Microbiol. 2025 Jun 9;15:1578518. doi: 10.3389/fcimb.2025.1578518. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40552122 Free PMC article.
-
Purification and characterization of soluble recombinant Crimean-Congo hemorrhagic fever virus glycoprotein Gc expressed in mammalian 293F cells.BMC Biotechnol. 2024 Aug 27;24(1):59. doi: 10.1186/s12896-024-00885-y. BMC Biotechnol. 2024. PMID: 39192233 Free PMC article.
-
Zoonotic arbovirus infections in cattle in Mozambique with special reference to Crimean-Congo hemorrhagic fever virus (CCHFV) and rift valley fever virus (RVFV).Virol J. 2025 Jun 6;22(1):185. doi: 10.1186/s12985-025-02804-9. Virol J. 2025. PMID: 40481487 Free PMC article.
-
Ribavirin for treating Crimean Congo haemorrhagic fever.Cochrane Database Syst Rev. 2018 Jun 5;6(6):CD012713. doi: 10.1002/14651858.CD012713.pub2. Cochrane Database Syst Rev. 2018. PMID: 29869797 Free PMC article.
-
Ticks infected with Crimean-Congo hemorrhagic fever virus (CCHFV): A decision approach systematic review and meta-analysis regarding their role as vectors.Travel Med Infect Dis. 2022 May-Jun;47:102309. doi: 10.1016/j.tmaid.2022.102309. Epub 2022 Mar 19. Travel Med Infect Dis. 2022. PMID: 35318129
References
-
- Gargili A., Estrada-Peña A., Spengler J.R., Lukashev A., Nuttall P.A., Bente D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017;144:93–119. doi: 10.1016/j.antiviral.2017.05.010. - DOI - PMC - PubMed
-
- Lindeborg M., Barboutis C., Ehrenborg C., Fransson T., Jaenson T.G., Lindgren P.E., Lundkvist A., Nyström F., Salaneck E., Waldenström J., et al. Migratory birds, ticks, and crimean-congo hemorrhagic fever virus. Emerg. Infect. Dis. 2012;18:2095–2097. doi: 10.3201/eid1812.120718. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

