Design and early progress of the Comparison of Anticoagulation and anti-Platelet Therapies for Intracranial Vascular Atherostenosis (CAPTIVA) trial
- PMID: 39862061
- PMCID: PMC12092178
- DOI: 10.1177/17474930241313301
Design and early progress of the Comparison of Anticoagulation and anti-Platelet Therapies for Intracranial Vascular Atherostenosis (CAPTIVA) trial
Abstract
Background: The usual antithrombotic treatment for symptomatic intracranial atherosclerotic stenosis (ICAS) consists of dual treatment with clopidogrel and aspirin for 90 days followed by aspirin alone but the risk of recurrent stroke remains high up to 12 months. The Comparison of Anticoagulation and anti-Platelet Therapies for Intracranial Vascular Atherostenosis (CAPTIVA) trial was designed to determine whether other combinations of dual antithrombotic therapy are superior to clopidogrel and aspirin.
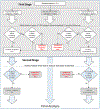
Methods: CAPTIVA is an ongoing, prospective, double-blinded, three-arm clinical trial at over 100 sites in the United States and Canada that will randomize 1683 high-risk subjects with a symptomatic infarct attributed to 70-99% stenosis of a major intracranial artery to 12 months of treatment with (1) ticagrelor (180 mg loading dose, then 90 mg twice daily), (2) low-dose rivaroxaban (2.5 mg twice daily), or (3) clopidogrel (600 mg loading dose, then 75 mg daily). All subjects receive aspirin (81 mg daily), intensive risk factor management, and will undergo blinded CYP2C19 genotype analysis. The primary goal of the trial is to determine whether rivaroxaban or ticagrelor or both are superior to clopidogrel for lowering the primary endpoint (ischemic stroke, intracerebral hemorrhage (ICH), or vascular death) within 12 months. A prespecified interim safety analysis will be conducted when the first 450 randomized subjects have been followed for 12 months to evaluate the risk of major hemorrhage in the rivaroxaban and ticagrelor arms.
Results: Enrollment began in August 2022 and, as of 26 June 2024, the 450th subject was randomized into the study.
Conclusion: CAPTIVA is evaluating two alternative dual antithrombotic therapies to clopidogrel and aspirin to maximize the chance of establishing more effective antithrombotic therapy for symptomatic ICAS, one of the most common and high-risk cerebrovascular diseases worldwide.
Keywords: Intracranial arteriosclerosis; anticoagulants; antiplatelet; ischemic stroke.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:B.L.H., MD, MBA:Relevant: NIH, AstraZeneca is supplying ticagrelor and Janssen Pharmaceutical is supplying rivaroxaban for the CAPTIVA trial.Not relevant: University of Florida, NIH, and research support from the Brain Aneurysm Foundation and The Aneurysm and AVM Foundation. B.L.H. is an investor in FastWave, Galaxy Therapeutics, Kandu, Progressive Neuro, ProprioVision, and Solenic.M. I. C., MBChB:Relevant: NIH. Salary support as a multiple principal investigator for the ongoing NIH-funded CAPTIVA trial. AstraZeneca is supplying ticagrelor and Janssen Pharmaceutical is supplying rivaroxaban for the CAPTIVA trial.Not relevant: Medtronic (DSMB for subdural hemorrhage trial), Boehringer Ingelheim (stroke adjudicator for steatohepatitis clinical trial)S.D.Y., PhD:Relevant: NIHNot Relevant: journal
Figures
References
-
- Gorelick PB, Wong KS, Bae HJ and Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008; 39: 2396–2399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

