Three-year treatment with anti-CGRP monoclonal antibodies modifies migraine course: the prospective, multicenter I-GRAINE study
- PMID: 39862304
- PMCID: PMC11762429
- DOI: 10.1007/s00415-025-12911-w
Three-year treatment with anti-CGRP monoclonal antibodies modifies migraine course: the prospective, multicenter I-GRAINE study
Abstract
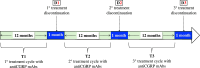
Objectives: To determine whether extending anti-CGRP mAb treatment beyond 3 years influences migraine course, we analyzed migraine frequency during the first month of treatment discontinuation following three 12-month treatment cycles (Ts).
Methods: This multicenter, prospective, real-world study enrolled 212 patients with high-frequency episodic migraine (HFEM) or chronic migraine (CM) who completed three consecutive Ts of subcutaneous anti-CGRP mAbs. Discontinuation periods (D1, D2, D3) were defined as the first month after T1, T2, and T3, respectively. The primary endpoint was the ≥ 50% response rate at D3 compared to D2. Secondary endpoints included changes in monthly migraine days (MMD), monthly headache days (MHD), monthly analgesic intake (MAI), numerical rating scale (NRS), Headache Impact Test-6 (HIT-6), ≥ 50% response rate at D3 versus D1 and D2, and relapse rates to CM or medication overuse.
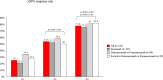
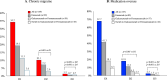
Results: At D3 vs. D2, significant improvements (p < 0.001) were observed in the ≥ 50% response rate (77.8% vs. 53.8%), MMD (- 2.1 ± 1.7), MHD (- 2.9 ± 2.4), MAI (- 2.6 ± 2.4), NRS (- 0.7 ± 1.3), and HIT-6 (- 7.2 ± 5.9), with lower relapse rates to CM (2.3% vs. 18%) and medication overuse (1.3% vs. 10.1%). Compared to D1, D3 demonstrated greater benefits (p < 0.001) in MMD (- 2.6 ± 1.9), MHD (- 5.8 ± 3.3), MAI (- 4.9 ± 3.4), NRS (- 1 ± 1.6), and HIT- 6 (- 9.4 ± 7), alongside higher ≥ 50% response rates (77.8% vs. 25%) and reduced relapses to CM (2.3% vs. 67.7%) and medication overuse (1.3% vs. 34.2%).
Discussion: Three years of anti-CGRP mAb treatment revealed a progressive increase in the proportion of ≥ 50% responders (D1: 25%; D2: 53.8%; D3: 77.8%) and substantial reductions in migraine burden, suggesting that prolonged treatment may favorably modify migraine course.
Keywords: Anti-CGRP mAbs; Discontinuation; Disease modifier; Migraine; Real world; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Piero Barbanti reports personal compensation for consulting, serving on a scientific advisory board, speaking, research support, collaborated for clinical trials, or other activities with Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, DOC Pharma, Eli-Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, Noema Pharma, Organon, Pfizer, Teva, Viatris, Visufarma, and Zambon, and serves as President with Italian Association of Headache Sufferers. Cinzia Aurilia received travel grants from Eli-Lilly, FB-Health, Lusofarmaco, and Teva, and honoraria from Novartis, Eli-Lilly, and Teva; Paola Torelli received travel grant, honoraria as a speaker, or for participating in advisory boards from Novartis, Teva, Eli Lilly, and Allergan; Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta, and Ecupharma; Florindo d’Onofrio received travel grant, honoraria as a speaker or for partecipating in advisory boards from Novartis, Teva, Neopharmed Gentili, Qbgroup srl, K link srl, and Eli-Lilly; Cinzia Finocchi received grants and honoraria from Novartis, Eli Lilly, TEVA, and AIM group; Giovanna Viticchi received honoraria for speaker activities and participating in advisory boards from Eli-lilly, AbbVie, Teva, and Boehringer Ingelheim; Marco Russo received travel grants and honoraria as a speaker or for participating in expert panels from Eli Lilly, Abbvie, and Lundbeck; Simone Quintana received travel grants and honoraria as a speaker or for participating in expert panels from Eli Lilly, Abbvie, and Pfizer; Roberta Messina received honoraria for speaker activities and participating in advisory boards from Abbvie, Biomedia, Eli Lilly, Lundbeck, Organon, Pfizer, and Teva; Marco Bartolini received honoraria for speaker activities from Lusofarmaco and Neopharmed; Massimo Filippi is the Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Horizon, Merck, Novartis, Roche, and Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Horizon, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Horizon, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, and Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, and Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA); Sabina Cevoli received honoraria for speaker panels from Teva and Novartis; Antonio Carnevale, Bianca Orlando, Giulia Fiorentini, Francesca Pistoia, Stefano Bonassi, and Alice Mannocci have no disclosures to declare.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

