Superior performance in classification of breast cancer molecular subtype and histological factors by radiomics based on ultrafast MRI over standard MRI: evidence from a prospective study
- PMID: 39862364
- PMCID: PMC11903601
- DOI: 10.1007/s11547-025-01956-6
Superior performance in classification of breast cancer molecular subtype and histological factors by radiomics based on ultrafast MRI over standard MRI: evidence from a prospective study
Abstract
Purpose: To compare the performance of ultrafast MRI with standard MRI in classifying histological factors and subtypes of invasive breast cancer among radiologists with varying experience.
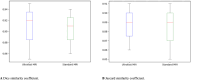
Methods: From October 2021 to November 2022, this prospective study enrolled 225 participants with 233 breast cancers before treatment (NCT06104189 at clinicaltrials.gov). Tumor segmentation on MRI was performed independently by two readers (R1, dedicated breast radiologist; R2, radiology resident). We extracted 1618 radiomic features and four kinetic features from ultrafast and standard images, respectively. Logistic regression algorithms were adopted for prediction modeling, following feature selection by the least absolute shrinkage and selection operator. The performance of predicting histological factors and subtypes was evaluated using the area under the receiver-operating characteristic curve (AUC). Performance differences between MRI methods and radiologists were assessed using the DeLong test.
Results: Ultrafast MRI outperformed standard MRI in predicting HER2 status (AUCs [95% CI] of ultrafast MRI vs standard MRI; 0.87 [0.83-0.91] vs 0.77 [0.64-0.90] for R1 and 0.88 [0.83-0.91] vs 0.77 [0.69-0.84] for R2) (all P < 0.05). Both ultrafast MRI and standard MRI showed comparable performance in predicting hormone receptors. Ultrafast MRI exhibited superior performance to standard MRI in classifying subtypes. The classification of the luminal subtype for both readers, the HER2-overexpressed subtype for R2, and the triple-negative subtype for R1 was significantly better with ultrafast MRI (P < 0.05).
Conclusion: Ultrafast MRI-based radiomics holds promise as a noninvasive imaging biomarker for classifying hormone receptors, HER2 status, and molecular subtypes compared to standard MRI, regardless of radiologist experience.
Keywords: Breast cancer; Histological factor; Magnetic resonance imaging; Radiomics; Subtype.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have declared no conflict of interest. Ethical approval: This prospective study was approved by the Institutional Review Board of Korea University Ansan Hospital (Approval No. 2021AS0318). This study was registered at clinicaltrials.gov (NCT06104189). Consent to participate: Written informed consent was obtained from all participants prior to data collection.
Figures




References
-
- Milon A, Vande Perre S, Poujol J, Trop I, Kermarrec E, Bekhouche A et al (2019) Abbreviated breast MRI combining FAST protocol and high temporal resolution (HTR) dynamic contrast enhanced (DCE) sequence. Eur J Radiol 117:199–208. 10.1016/j.ejrad.2019.06.022 - PubMed
-
- Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292(3):520–536. 10.1148/radiol.2019182947 - PubMed
-
- Ramtohul T, Tescher C, Vaflard P, Cyrta J, Girard N, Malhaire C et al (2022) Prospective evaluation of ultrafast breast MRI for predicting pathologic response after neoadjuvant therapies. Radiology 305(3):565–574. 10.1148/radiol.220389 - PubMed
-
- Oldrini G, Fedida B, Poujol J, Felblinger J, Trop I, Henrot P et al (2017) Abbreviated breast magnetic resonance protocol: Value of high-resolution temporal dynamic sequence to improve lesion characterization. Eur J Radiol 95:177–185. 10.1016/j.ejrad.2017.07.025 - PubMed
-
- Honda M, Kataoka M, Iima M, Miyake KK, Ohashi A, Kishimoto AO et al (2020) Background parenchymal enhancement and its effect on lesion detectability in ultrafast dynamic contrast-enhanced MRI. Eur J Radiol 129:108984. 10.1016/j.ejrad.2020.108984 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

