Ferroptosis triggers mitochondrial fragmentation via Drp1 activation
- PMID: 39863602
- PMCID: PMC11762985
- DOI: 10.1038/s41419-024-07312-2
Ferroptosis triggers mitochondrial fragmentation via Drp1 activation
Abstract
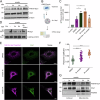
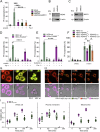
Constitutive mitochondrial dynamics ensure quality control and metabolic fitness of cells, and their dysregulation has been implicated in various human diseases. The large GTPase Dynamin-related protein 1 (Drp1) is intimately involved in mediating constitutive mitochondrial fission and has been implicated in mitochondrial cell death pathways. During ferroptosis, a recently identified type of regulated necrosis driven by excessive lipid peroxidation, mitochondrial fragmentation has been observed. Yet, how this is regulated and whether it is involved in ferroptotic cell death has remained unexplored. Here, we provide evidence that Drp1 is activated upon experimental induction of ferroptosis and promotes cell death execution and mitochondrial fragmentation. Using time-lapse microscopy, we found that ferroptosis induced mitochondrial fragmentation and loss of mitochondrial membrane potential, but not mitochondrial outer membrane permeabilization. Importantly, Drp1 accelerated ferroptotic cell death kinetics. Notably, this function was mediated by the regulation of mitochondrial dynamics, as overexpression of Mitofusin 2 phenocopied the effect of Drp1 deficiency in delaying ferroptosis cell death kinetics. Mechanistically, we found that Drp1 is phosphorylated and activated after induction of ferroptosis and that it translocates to mitochondria. Further activation at mitochondria through the phosphatase PGAM5 promoted ferroptotic cell death. Remarkably, Drp1 depletion delayed mitochondrial and plasma membrane lipid peroxidation. These data provide evidence for a functional role of Drp1 activation and mitochondrial fragmentation in the acceleration of ferroptotic cell death, with important implications for targeting mitochondrial dynamics in diseases associated with ferroptosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations concerning good scientific practice. The study did not require ethical approval as it did not include live vertebrates or human participants.
Figures


References
-
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. https://pubmed.ncbi.nlm.nih.gov/22632970. - PMC - PubMed
-
- Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22:1520–30. https://pubmed.ncbi.nlm.nih.gov/28886009/. - PubMed
-
- Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99. https://pubmed.ncbi.nlm.nih.gov/30737476/. - PMC - PubMed
-
- Conrad M, Angeli JPF, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. 15, Nature Reviews Drug Discovery. Nature Publishing Group; 2016 [cited 2020 Oct 11]: [348–66 p]. Available from: https://pubmed.ncbi.nlm.nih.gov/26775689/. - PMC - PubMed
-
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. Cell Press. 2017;171:273–85. https://pubmed.ncbi.nlm.nih.gov/28985560/. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

