Effect of pocket compression device on pocket hematoma after cardiac implantable electronic device implantation
- PMID: 39863676
- PMCID: PMC11762974
- DOI: 10.1038/s41598-025-87735-9
Effect of pocket compression device on pocket hematoma after cardiac implantable electronic device implantation
Abstract
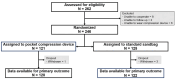
Pocket hematoma is a common and serious complication following cardiac implantable electronic device (CIED) implantation, contributing to significant morbidity and mortality. This study aimed to evaluate the efficacy of a novel pocket compression device in reducing pocket hematoma occurrence. We enrolled 242 patients undergoing CIED implantation, randomly assigning them to receive either the novel compression vest with a pressure cuff or conventional sandbag compression. Pocket hematomas were categorized as grade 1 (mild), grade 2 (moderate), or grade 3 (severe, requiring intervention or prolonged hospitalization). The primary endpoint, incidence of pocket hematoma 24 h post-procedure, did not significantly differ between the two groups (26.7% vs. 19.7%, p = 0.224). Rates of grade 2 hematoma were similarly low and comparable (2.5% vs. 0.8%, p = 0.368), with no grade 3 hematomas observed. Skin reactions and patient comfort were similar between groups. The sole predictor for hematoma occurrence was current oral anticoagulation use. In conclusion, our study found a low incidence of clinically significant pocket hematomas. The novel pocket compression device showed comparable efficacy to conventional methods, suggesting it as a viable alternative for reducing post-procedural complications without additional adverse effects.Trial registration number: The study was registered in the Thai Clinical Trials Registry (TCTR) at https://www.thaiclinicaltrials.org/ , with the identification number TCTR20230913005, date of first trial registration 13/09/2023.
Keywords: Cardiac implantable electronic devices; Defibrillator; Pacemaker; Pocket compression; Pocket hematoma..
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Glikson, M. et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Gr. Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 24, 71–164. 10.1093/europace/euab232 (2022).
-
- Al-Khatib, S. M. et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the Prevention of Sudden Cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol.72, e91–e220. 10.1016/j.jacc.2017.10.054 (2018). - PubMed
-
- Awada, H., Geller, J. C., Brunelli, M. & Ohlow, M. A. Pocket related complications following cardiac electronic device implantation in patients receiving anticoagulation and/or dual antiplatelet therapy: prospective evaluation of different preventive strategies. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing. 54, 247–255. 10.1007/s10840-018-0488-y (2019). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

