Osilodrostat improves blood pressure and glycemic control in patients with Cushing's disease: a pooled analysis of LINC 3 and LINC 4 studies
- PMID: 39863744
- PMCID: PMC11762609
- DOI: 10.1007/s11102-024-01471-3
Osilodrostat improves blood pressure and glycemic control in patients with Cushing's disease: a pooled analysis of LINC 3 and LINC 4 studies
Erratum in
-
Correction to: Osilodrostat improves blood pressure and glycemic control in patients with Cushing's disease: a pooled analysis of LINC 3 and LINC 4 studies.Pituitary. 2025 May 21;28(3):62. doi: 10.1007/s11102-025-01525-0. Pituitary. 2025. PMID: 40399732 Free PMC article. No abstract available.
Abstract
Purpose: To evaluate the effect of osilodrostat and hypercortisolism control on blood pressure (BP) and glycemic control in patients with Cushing's disease.
Methods: Pooled analysis of two Phase III osilodrostat studies (LINC 3 and LINC 4), both comprising a 48-week core phase and an optional open-label extension. Changes from baseline in systolic and diastolic BP (SBP and DBP), fasting plasma glucose (FPG), and glycated hemoglobin (HbA1c) were evaluated during osilodrostat treatment in patients with/without hypertension or diabetes at baseline.
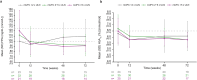
Results: Of 210 patients, 82.9% met criteria for hypertension and 40.0% for diabetes at baseline. In patients with hypertension, reductions in mean SBP/DBP were observed from week (W)12 to W72, and 49.1%/58.5% of patients with high SBP/DBP (> 130/>90 mmHg) at baseline had normotensive levels at W72. Antihypertensive medication dose was reduced/stopped in 26.8% of patients, and the proportion taking antihypertensive medication decreased from 54.3% at baseline to 47.3% at W72. In patients with diabetes, mean FPG and HbA1c decreased from W12 to W72, and 33.3%/61.5% with high FPG/HbA1c (≥ 100 mg/dL/≥6.5%) at baseline had normal levels at W72. Antihyperglycemic medication dose was reduced/stopped in 35.7% of patients, and the proportion taking antihyperglycemic medication decreased from 21.9% at baseline to 17.1% at W72; improvements in SBP/DBP and FPG/HbA1c were correlated with improvement in mean urinary free cortisol but not weight change. BP/glycemic parameters generally remained normal in patients without hypertension/diabetes at baseline.
Conclusions: Patients with Cushing's disease and comorbid hypertension/diabetes receiving osilodrostat had rapid and sustained improvements in SBP/DBP and glycemic control, respectively.
Keywords: Cortisol normalization; Cushing’s disease; Diabetes; Hypertension; Long-term treatment; Osilodrostat.
Plain language summary
WHY WAS THIS ANALYSIS CARRIED OUT?: People with Cushing’s syndrome have higher-than-normal levels of the hormone cortisol. Cushing’s disease, the most common form of Cushing’s syndrome, is caused by a benign adenoma (non-cancerous tumor) of the pituitary gland. The adenoma produces too much of a hormone called adrenocorticotropic hormone (ACTH), which controls the production of cortisol. Osilodrostat is a medicine that rapidly reduces and maintains normal cortisol levels in people with Cushing’s syndrome, including Cushing’s disease. Many people with Cushing’s disease also have other medical conditions caused by high cortisol levels, such as high blood pressure and diabetes. We wanted to examine whether treatment with osilodrostat has any effect on blood pressure and blood sugar (glucose) levels in patients with Cushing’s disease. HOW WAS THIS ANALYSIS CARRIED OUT?: The results of two previous studies of osilodrostat were combined to allow a larger number of people to be included over a long period. Changes in blood pressure and glucose levels were measured during treatment with osilodrostat. WHAT WERE THE OVERALL RESULTS?: In patients who had high blood pressure (hypertension) before starting osilodrostat, there was an overall reduction in blood pressure during osilodrostat treatment. Some people who were taking medication for their hypertension were able to reduce the dose of their medication or stop it completely. In patients who had normal blood pressure before starting osilodrostat, blood pressure remained normal during osilodrostat treatment. In patients who had diabetes before starting osilodrostat, there was an overall reduction in glucose levels during osilodrostat treatment. Some people who were taking medication for their diabetes were able to reduce the dose of their medication or stop it completely. In patients with normal glucose levels before starting osilodrostat, glucose levels remained normal during osilodrostat treatment. WHAT DO THE RESULTS MEAN?: By lowering cortisol levels, osilodrostat improves blood pressure in patients with hypertension and glucose levels in patients with diabetes; these effects were sustained over the long term. This reduces the overall burden of disease for patients and may partly explain the improvement in quality of life reported in patients with Cushing’s disease treated with osilodrostat. WHERE CAN I FIND MORE INFORMATION?: LINC 3 primary publication: Pivonello R et al. Lancet Diabetes Endocrinol 2020;8:748–61. LINC 4 primary publication: Gadelha M et al. J Clin Endocrinol Metab 2022;107:e2882–95. LINC 3 long-term publication: Fleseriu M et al. Eur J Endocrinol 2022;187:531−41. LINC 4 long-term publication: Gadelha M et al. Front Endocrinol (Lausanne) 2023;14:1236465. LINC 4 plain-language summary publication: Gadelha M. Future Rare Dis 2022;2:FRD28.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The LINC 3 and LINC 4 studies were conducted in accordance with the Declaration of Helsinki, and study protocols were approved by independent ethics committees/institutional review boards. Consent to participate: Patients provided written informed consent to participate at the start of the core and extension phases of the studies. Consent to publish: Patients provided written informed consent for their data to be published anonymously. Competing interests: MF reports grants to her university from Crinetics and Sparrow and occasional scientific consulting fees from Crinetics, Recordati Rare Diseases, Sparrow, and Xeris Pharmaceuticals; she served as a member of the LINC 3 steering committee and is a member of the editorial board of Pituitary. RP has received research funding from Recordati AG, Corcept Therapeutics, Xeris Pharmaceuticals (Strongbridge), Neurocrine Biosciences, and Spruce Biosciences and has served as a consultant for Corcept Therapeutics, Recordati AG, Crinetics Pharmaceuticals, and H Lundbeck A/S. JN-P reports research grants and consultancy payments to his university from Crinetics, Diurnal, HRA Pharma, and Recordati Rare Diseases. MRG has received speaker fees from Recordati, Ipsen, Novo Nordisk, and Camurus and attended advisory boards for Novo Nordisk, Recordati, and Crinetics Pharmaceuticals. BMKB reports occasional consulting honoraria from H Lundbeck A/S, Recordati Rare Diseases, Sparrow, and Xeris Pharmaceuticals; she served on the LINC 3 steering committee. RJA reports grants and personal fees from Xeris Pharmaceuticals, Spruce Biosciences, Neurocrine Biosciences, Corcept Therapeutics, Diurnal Ltd, Sparrow Pharmaceuticals, Crinetics Pharmaceuticals, and Recordati Rare Diseases and personal fees from Adrenas Therapeutics, Quest Diagnostics, H Lundbeck A/S, Novo Nordisk, and Besins Pharmaceuticals. RF reports consultancy for Corcept Therapeutics and Recordati and research grants from Strongbridge and Corcept Therapeutics. AS reports serving as a speaker and consultant for Recordati; he was also a member of the LINC 3 steering committee. PW reports receiving travel grants and speaker fees from Novartis, Ipsen, Recordati, Novo Nordisk, Strongbridge Biopharma, Merck-Serono, Lilly, and Berlin Chemie. MB reports consultancy for and travel grants from Recordati. AP is an employee of Recordati. AMP was an employee of Recordati at the time the analyses were conducted. AL reports grants and personal consulting fees from Novartis, Recordati, Pfizer, and Corcept Therapeutics.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

