TET1 participates in oxaliplatin-induced neuropathic pain by regulating microRNA-30b/Nav1.6
- PMID: 39864621
- PMCID: PMC11894311
- DOI: 10.1016/j.jbc.2025.108228
TET1 participates in oxaliplatin-induced neuropathic pain by regulating microRNA-30b/Nav1.6
Abstract
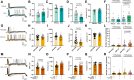
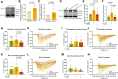
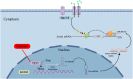
Chemotherapy-induced neuropathic pain poses significant clinical challenges and severely impacts patient quality of life. Sodium ion channels are crucial in regulating neuronal excitability and pain. Our research indicates that the microRNA-30b (miR-30b) in rat dorsal root ganglia (DRG) contributes to chemotherapy-induced neuropathic pain by regulating the Nav1.6 protein. Additionally, ten-eleven translocation methylcytosine dioxygenase 1 (TET1) plays a crucial role in pain generation by altering gene expression. We established a chemotherapy-induced neuropathy model using intraperitoneal oxaliplatin (OXA) injections and measured TET1 and Nav1.6 protein in the DRG. Using lentivirus and Tet1flox/flox mice, we modulated TET1 expression and assessed pain behaviors, DRG neuronal excitability, Nav1.6 currents, miR-30b-5p, and demethylation of the Mir30b promoter region. We employed chromatin immunoprecipitation to pinpoint TET1-binding sites on the Mir30b promoter. The impacts of miR-30b agomir or antagomir on Nav1.6 expression and pain responses were assessed postintrathecal injections. The results showed that OXA reduced TET1, increasing neuronal excitability, Nav1.6 currents, and miR-30b-5p in the DRG. TET1 knockdown exacerbated these effects and induced pain behaviors. Conversely, TET1 overexpression reversed these effects. TET1 also targeted and enhanced demethylation at the Mir30b promoter (-1103 bp to -1079 bp). miR-30b agomir reduces Nav1.6, whereas miR-30b antagomir reverses TET1's effects on Nav1.6 and pain. In OXA-induced neuropathy, decreased TET1 reduces miR-30b, elevating Nav1.6 expression and currents and contributing to pain. We hypothesize that TET1 mediates this process by regulating the demethylation of the Mir30b promoter.
Keywords: 5-hydroxymethylcytosine; DRG; Nav1.6; TET1; epigenetics; microRNA; oxaliplatin; pain; sodium channels.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Starobova H., Mueller A., Deuis J.R., Carter D.A., Vetter I. Inflammatory and neuropathic gene expression signatures of chemotherapy-induced neuropathy induced by vincristine, cisplatin, and oxaliplatin in C57BL/6J mice. J. Pain. 2020;21:182–194. - PubMed
-
- Shidahara Y., Natsume T., Awaga Y., Ogawa S., Yamoto K., Okamoto S., et al. Distinguishing analgesic drugs from non-analgesic drugs based on brain activation in macaques with oxaliplatin-induced neuropathic pain. Neuropharmacology. 2019;149:204–211. - PubMed
-
- Mauri G., Gori V., Bonazzina E., Amatu A., Tosi F., Bencardino K., et al. Oxaliplatin retreatment in metastatic colorectal cancer: systematic review and future research opportunities. Cancer Treat Rev. 2020;91 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

