Persistent effect of salt reduction in schoolchildren and their families: 1-year follow-up after an application-based cluster randomized controlled trial
- PMID: 39865267
- PMCID: PMC11771009
- DOI: 10.1186/s12916-025-03868-8
Persistent effect of salt reduction in schoolchildren and their families: 1-year follow-up after an application-based cluster randomized controlled trial
Abstract
Background: A 12-month cluster randomized controlled trial (RCT) demonstrated the effectiveness of an application-based education program in reducing the salt intake and systolic blood pressure (SBP) of schoolchildren's adult family members. This study aimed to assess whether the effect at 12 months persisted at 24 months.
Methods: Fifty-four schools were randomly assigned to either the intervention or control group. All participants (594 children in grade 3 and 1188 of their adult family members) who completed the baseline survey were contacted again 12 months after the trial. The primary outcome was the difference in salt intake change between the intervention and control groups at 24 months versus baseline and 12 months, measured by the mean two consecutive 24-h urinary sodium excretions. The secondary outcome was the difference in the change of blood pressure and salt-related Knowledge, Attitude, Practice (KAP) score.
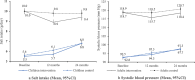
Results: The difference in salt intake change in adults between the intervention and control groups after adjusting for confounding factors was - 0.38 g/day at 24 months versus baseline (95% CI - 0.81 to 0.05, p = 0.09), following the - 0.83 g/day (95% CI - 1.25 to - 0.41, p < 0.001) at 12 months. The adjusted difference in SBP change was - 2.19 mm Hg (95% CI - 3.63 to - 0.76, p = 0.003) at 24 months versus baseline, following the - 1.80 mm Hg (95% CI - 3.19 to - 0.40, p = 0.01) at 12 months. The intervention group had a higher KAP score than the control group both at 12 months and at 24 months versus baseline. No significant changes were found in children.
Conclusions: The effect of the education program on adults' salt intake faded, but the SBP lowering effect and the improvement of KAP score remained 12 months after the completion of the RCT. Continuous efforts are needed to maintain the salt reduction effects in real-world settings.
Trial registration: ChiCTR1800017553. Registered on August 3, 2018.
Keywords: Blood pressure; Follow-up; Persistent effect; Salt reduction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Queen Mary (University of London) Ethics of Research Committee (QMERC2018/13) and the Peking University Institutional Review Board in China (IRB00001052-18051). Permissions were obtained from the local education authorities and school headteachers. All participants who took part in the outcome assessments gave written informed consent. For children, participant assent and parental written consent were obtained. Consent for publication: Not applicable. Competing interests: FJH is an unpaid member of Action on Salt and World Action on Salt, Sugar and Health (WASSH); no other relationships or activities that could appear to have influenced the submitted work. All other authors declare no competing interests.
Figures
Similar articles
-
Effectiveness of an mHealth- and School-Based Health Education Program for Salt Reduction (EduSaltS) in China: Cluster Randomized Controlled Trial Within Scale-Up.J Med Internet Res. 2025 Mar 27;27:e60092. doi: 10.2196/60092. J Med Internet Res. 2025. PMID: 40017342 Free PMC article. Clinical Trial.
-
School based education programme to reduce salt intake in children and their families (School-EduSalt): cluster randomised controlled trial.BMJ. 2015 Mar 18;350:h770. doi: 10.1136/bmj.h770. BMJ. 2015. PMID: 25788018 Free PMC article. Clinical Trial.
-
App based education programme to reduce salt intake (AppSalt) in schoolchildren and their families in China: parallel, cluster randomised controlled trial.BMJ. 2022 Feb 9;376:e066982. doi: 10.1136/bmj-2021-066982. BMJ. 2022. PMID: 35140061 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effect of salt reduction interventions in lowering blood pressure in Chinese populations: a systematic review and meta-analysis of randomised controlled trials.BMJ Open. 2020 Feb 17;10(2):e032941. doi: 10.1136/bmjopen-2019-032941. BMJ Open. 2020. PMID: 32071177 Free PMC article.
References
-
- Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1223–49. 10.1016/s0140-6736(20)30752-2. - PMC - PubMed
-
- World Health Organization. Tackling NCDs: “best buys” and other recommended interventions for the prevention and control of noncommunicable diseases. https://www.who.int/publications/i/item/WHO-NMH-NVI-17.9. Accessed 26 October 2017.
-
- World Health Organization. WHO global report on sodium intake reduction. https://www.who.int/publications-detail-redirect/97892400699859. Accessed 9 March 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical