CASC8 activates the pentose phosphate pathway to inhibit disulfidptosis in pancreatic ductal adenocarcinoma though the c-Myc-GLUT1 axis
- PMID: 39865281
- PMCID: PMC11771065
- DOI: 10.1186/s13046-025-03295-w
CASC8 activates the pentose phosphate pathway to inhibit disulfidptosis in pancreatic ductal adenocarcinoma though the c-Myc-GLUT1 axis
Abstract
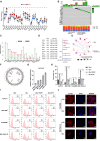
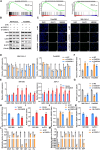
Purpose: Glucose starvation induces the accumulation of disulfides and F-actin collapse in cells with high expression of SLC7A11, a phenomenon termed disulfidptosis. This study aimed to confirm the existence of disulfidptosis in pancreatic ductal adenocarcinoma (PDAC) and elucidate the role of Cancer Susceptibility 8 (CASC8) in this process.
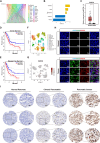
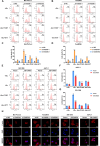
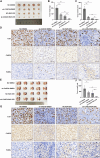
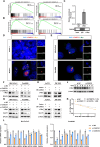
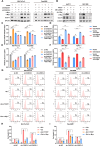
Methods: The existence of disulfidptosis in PDAC was assessed using flow cytometry and F-actin staining. CASC8 expression and its clinical correlations were analyzed using data from The Cancer Genome Atlas (TCGA) and further verified by chromogenic in situ hybridization assay in PDAC tissues. Cells with CASC8 knockdown and overexpression were subjected to cell viability, EdU, transwell assays, and used to establish subcutaneous and orthotopic tumor models. Disulfidptosis was detected by flow cytometry and immunofluorescence assays. RNA sequencing and metabolomics analysis were performed to determine the metabolic pathways which were significantly affected after CASC8 knockdown. We detected the glucose consumption and the NADP+/NADPH ratio to investigate alterations in metabolic profiles. RNA immunoprecipitation combined with fluorescence in situ hybridization assay was used to identify protein-RNA interactions. Protein stability, western blotting and quantitative real-time PCR assays were performed to reveal potential molecular mechanism.
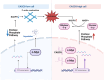
Results: Disulfidptosis was observed in PDAC and could be significantly rescued by disulfidptosis inhibitors. CASC8 expression was higher in PDAC samples compared to normal pancreatic tissue. High CASC8 expression correlated with a poor prognosis for patients with PDAC and contributed to cancer progression in vitro and in vivo. Furthermore, CASC8 was associated with disulfidptosis resistance under glucose starvation conditions in PDAC. Mechanistically, CASC8 interacted with c-Myc to enhance the stability of c-Myc protein, leading to the activation of the pentose phosphate pathway, a reduction of the NADP+/NADPH ratio and ultimately inhibiting disulfidptosis under glucose starvation conditions.
Conclusions: This study provides evidence for the existence of disulfidptosis in PDAC and reveals the upregulation of CASC8 in this malignancy. Furthermore, we demonstrate that CASC8 acts as a crucial regulator of the pentose phosphate pathway and disulfidptosis, thereby promoting PDAC progression.
Keywords: Cancer susceptibility 8; Disulfidptosis; Pancreatic ductal adenocarcinoma; Pentose phosphate pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures related to patients were carried out in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS). Consent for publication: All authors had agreed to publish this manuscript. Competing interests: The authors have declared that no conflict of interest exists.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Sang W, Zhou Y, Chen H, Yu C, Dai L, Liu Z, Chen L, Fang Y, Ma P, Wu X, et al. Receptor-interacting Protein Kinase 2 Is an Immunotherapy Target in Pancreatic Cancer. Cancer Discov. 2024;14(2):326–47. - PubMed
-
- Zhang S, Yao HF, Li H, Su T, Jiang SH, Wang H, Zhang ZG, Dong FY, Yang Q, Yang XM. Transglutaminases are oncogenic biomarkers in human cancers and therapeutic targeting of TGM2 blocks chemoresistance and macrophage infiltration in pancreatic cancer. Cell Oncol (Dordr). 2023;46(5):1473–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

