RADAR-AD: assessment of multiple remote monitoring technologies for early detection of Alzheimer's disease
- PMID: 39865315
- PMCID: PMC11771057
- DOI: 10.1186/s13195-025-01675-0
RADAR-AD: assessment of multiple remote monitoring technologies for early detection of Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is a progressive neurodegenerative disorder affecting millions worldwide, leading to cognitive and functional decline. Early detection and intervention are crucial for enhancing the quality of life of patients and their families. Remote Monitoring Technologies (RMTs) offer a promising solution for early detection by tracking changes in behavioral and cognitive functions, such as memory, language, and problem-solving skills. Timely detection of these symptoms can facilitate early intervention, potentially slowing disease progression and enabling appropriate treatment and care.
Methods: The RADAR-AD study was designed to evaluate the accuracy and validity of multiple RMTs in detecting functional decline across various stages of AD in a real-world setting, compared to standard clinical rating scales. Our approach involved a univariate analysis using Analysis of Covariance (ANCOVA) to analyze individual features of six RMTs while adjusting for variables such as age, sex, years of education, clinical site, BMI and season. Additionally, we employed four machine learning classifiers - Logistic Regression, Decision Tree, Random Forest, and XGBoost - using a nested cross-validation approach to assess the discriminatory capabilities of the RMTs.
Results: The ANCOVA results indicated significant differences between healthy and AD subjects regarding reduced physical activity, less REM sleep, altered gait patterns, and decreased cognitive functioning. The machine-learning-based analysis demonstrated that RMT-based models could identify subjects in the prodromal stage with an Area Under the ROC Curve of 73.0 %. In addition, our findings show that the Amsterdam iADL questionnaire has high discriminatory abilities.
Conclusions: RMTs show promise in AD detection already in the prodromal stage. Using them could allow for earlier detection and intervention, thereby improving patients' quality of life. Furthermore, the Amsterdam iADL questionnaire holds high potential when employed remotely.
Keywords: Alzheimer’s disease; Discriminative capacity; Mobile applications; Remote monitoring technologies; Wearables.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The RADAR-AD was ethically approved in each participating country and aligns with the Helsinki Declaration of 1975, revised in 2008. All participants and their informants provided written informed consent prior to any study procedures. Competing interests: Authors AA, AKB, AM, CH, DR, GF, GW, HF, IL, LH, MGk, MGr, ML, MTG, PC, SN, VA, and VN declare no financial or non-financial competing interests. DA has received research support and/ or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, Evonik, Roche Diagnostics, and GE Health, and served as paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura, Eli Lilly, Cognetivity, Enterin, Acadia, EIP Pharma, and Biogen. JC is an employee and a shareholder of Novartis. GS is an employee of Novartis. NC is employed by Novartis Pharma AG, Basel, Switzerland. Research of Alzheimer center Amsterdam (MM, CB) is part of the neurodegeneration research program of Amsterdam Neuroscience. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting Steun Alzheimercentrum Amsterdam. MB is an employee of the Ace Alzheimer Center and an advisory board member for Grifols, Roche, Eli Lilly, Araclon Biotech, Merck, Zambon, Biogen, Novo Nordisk, Bioiberica, Eisai, Servier, and Schwabe Pharma. SG declares support for this work through the Italian Ministry of Health (Ricerca Corrente). GW and SV are employees and shareholders of Johnson & Johnson.
Figures

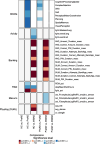
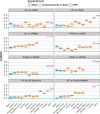
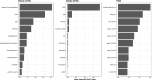
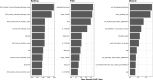
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

