Circulating Tumor DNA as a Marker of Recurrence Risk in Stage III Colorectal Cancer: The α-CORRECT Study
- PMID: 39865324
- PMCID: PMC12302971
- DOI: 10.1002/jso.27989
Circulating Tumor DNA as a Marker of Recurrence Risk in Stage III Colorectal Cancer: The α-CORRECT Study
Abstract
Background and objectives: Identification of colorectal cancer (CRC) patients at high risk of recurrence could be of substantial clinical use. We evaluated the association of ctDNA status, using a tumor-informed assay, with recurrence-free survival (RFS).
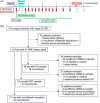
Methods: Stage III CRC patients were enrolled between 2016 and 2020. Tumor tissue and serial (every 3 months for years 1-3, biannually for years 4-5) blood samples were collected. Utilizing whole-exome sequencing and selection of 50-200 variants for tumor informed assays, ctDNA status was determined using plasma cell-free DNA.
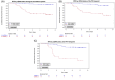
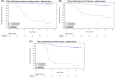
Results: Of 137 patients enrolled, 124 with 1029 ctDNA results were included in the analyses. Median follow-up was 4.8 years. Plasma ctDNA status was strongly associated with risk of recurrence during the surveillance period (hazard ratio (HR) 49.6, 95% CI: 16.6-148.3; p < 0.0001), and at the postsurgical (HR 9.6, 95% CI: 3.2-29.5) and postdefinitive therapy timepoints (HR: 16.7, 95% CI: 6.9-40.3). The estimated 3-year RFS for ctDNA positive and ctDNA negative patients were, respectively, 54.5% and 96.1% after surgery, and 18.2% and 90.0% after definitive therapy. Multivariable analysis indicated ctDNA but not CEA was strongly prognostic for recurrence.
Conclusions: Our tumor-informed ctDNA assay was strongly prognostic for recurrence in patients with stage III colorectal cancer at all timepoints.
Keywords: colorectal cancer; ctDNA; molecular residual disease (MRD); tumor‐informed.
© 2024 Exact Sciences Corporation and The Author(s). Journal of Surgical Oncology published by Wiley Periodicals LLC.
Conflict of interest statement
All authors except BD and RS are employees and stockholders of Exact Sciences. RS reports research support from Exact Sciences, Freenome and Immunovia, and advisory support from Guardant. BD declares no potential conflicts.
Figures

References
-
- Sung H., Ferlay J., Siegel R. L., et al., “Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 71, no. 3 (2021): 209–249. - PubMed
-
- André T., Boni C., Mounedji‐Boudiaf L., et al., “Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer,” New England Journal of Medicine 350, no. 23 (2004): 2343–2351. - PubMed
-
- André T., Boni C., Navarro M., et al., “Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial,” Journal of Clinical Oncology 27, no. 19 (2009): 3109–3116. - PubMed
-
- Gomez D., Calderón C., Carmona‐Bayonas A., et al., “Impact of Adjuvant Therapy Toxicity on Quality of Life and Emotional Symptoms in Patients With Colon Cancer: A Latent Class Analysis,” Clinical and Translational Oncology 23, no. 3 (2021): 657–662. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

