A preliminary ex vivo diffusion tensor imaging study of distinct aortic morphologies
- PMID: 39865441
- PMCID: PMC11996718
- DOI: 10.1111/joa.14223
A preliminary ex vivo diffusion tensor imaging study of distinct aortic morphologies
Abstract
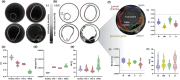
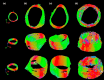
Changes in the microstructure of the aortic wall precede the progression of various aortic pathologies, including aneurysms and dissection. Current clinical decisions with regards to surgical planning and/or radiological intervention are guided by geometric features, such as aortic diameter, since clinical imaging lacks tissue microstructural information. The aim of this proof-of-concept work is to investigate a non-invasive imaging method, diffusion tensor imaging (DTI), in ex vivo aortic tissue to gain insights into the microstructure. This study examines healthy, aneurysm and a type B chronic dissection aortae, via DTI. DTI-derived metrics, such as the fractional anisotropy, mean diffusivity, helical angle and tractography, were examined in each morphology. The results from this work highlighted distinct differences in fractional anisotropy (healthy, 0.24 ± 0.008; aneurysmal, 0.19 ± 0.002; dissected, 0.13 ± 0.006) and a larger variation in the helical angle in the dissected aorta compared to healthy (39.28 ± 11.93° vs. 26.12 ± 4.60°, respectively). These differences were validated by histological characterisation. This study demonstrates the sensitivity of DTI to pathological changes in aortic tissue, highlighting the potential of this methodology to provide improved clinical insight.
Keywords: aneurysm; aortic disease; diffusion tensor imaging; dissection; magnetic resonance imaging.
© 2025 The Author(s). Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures




References
-
- Akin, I. (2020) Prediction of aortic dissection. Heart, 106, 870–871. - PubMed
-
- Bavaria, J.E. , Appoo, J.J. , Makaroun, M.S. , Verter, J. , Yu, Z.F. , Mitchell, R.S. et al. (2007) Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low‐risk patients: a multicenter comparative trial. The Journal of Thoracic and Cardiovascular Surgery, 133, 369–377. Available from: 10.1016/j.jtcvs.2006.07.040 - DOI - PubMed
-
- Bossone, E. & Eagle, K.A. (2021) Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nature Reviews Cardiology, 18, 331–348. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

