Real-world treatment patterns and health outcomes for patients with myelofibrosis treated with fedratinib
- PMID: 39865562
- PMCID: PMC11845104
- DOI: 10.1080/14796694.2025.2454895
Real-world treatment patterns and health outcomes for patients with myelofibrosis treated with fedratinib
Abstract
Aim: Assess real-world fedratinib (FEDR) treatment patterns and clinical outcomes in patients with primary or secondary myelofibrosis following discontinuation of ruxolitinib (RUX).
Patients & methods: This study was a retrospective, noninterventional medical record review of patients in Canada, Germany, and the United Kingdom (UK). A total of 70 physicians (primarily hematologist-oncologists [78.6%]) provided data for 196 eligible patients.
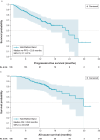
Results: Patients were mostly male (62.8%) with primary myelofibrosis (76.5%) and initiated FEDR at a mean age of 67.7 years. Median treatment duration was 11.5 months (median follow-up, 13.8 months), and nearly half (49.5%) of patients initiated FEDR at the label-indicated dose of 400 mg daily. Six months post-initiation, 77.7% and 66.8% of patients experienced symptom and spleen response, respectively. Kaplan-Meier estimates of median progression-free and overall survival from initiation were 23.8 months (95% CI, 21.1-27.6) and 29.8 months (95% CI, 23.9-NE), respectively.
Conclusion: These findings demonstrate real-world FEDR effectiveness among patients with myelofibrosis who discontinued RUX.
Keywords: Myelofibrosis; fedratinib; medical record review; real-world evidence; ruxolitinib; survival analysis.
Plain language summary
What is this summary about?Myelofibrosis (MF) is a rare blood cancer that can cause unhealthy spleen growth and symptoms, such as feeling tired, loss of appetite, bone pain, and fever. This is a summary of an article that reviewed medical records of patients with MF from treatment centers in Canada, Germany, and the United Kingdom (UK). The study looked at people who had been taking a medication called fedratinib (FEDR) for their MF after they had stopped taking a different medication called ruxolitinib (RUX). Many of the people stopped taking RUX because their MF got worse within a few years. The study wanted to see if taking FEDR reduced symptoms and spleen size for people with MF after they stopped taking RUX.What were the results?After at least 6 months of taking FEDR, 77.7% of the people in the study had fewer symptoms, and 66.8% of people in the study had a decrease in spleen size or no spleen growth. Additionally, most people taking FEDR after discontinuing RUX went nearly 2 years without their MF symptoms or illness getting worse.What do the results mean?These results suggest that FEDR is an effective treatment for people with MF who have stopped taking RUX.
Conflict of interest statement
MC, AY, SJ, DZ, and SS are employees of Bristol Myers Squibb. SK, RCP, JR, and KLD are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which was retained by Bristol Myers Squibb to conduct the research that is the subject of this manuscript. Their compensation is unconnected to the studies on which they work. Medical writing support was provided by Gabrielle Dardis, PhD, and Madison Walker, MPH, of RTI Health Solutions, and editing support was provided by John Forbes of RTI Health Solutions, as part of a contract with Bristol Myers Squibb.
Figures
References
-
- O’Sullivan JM, Harrison CN.. Myelofibrosis: clinicopathologic features, prognosis, and management. Clin Adv Hematol Oncol. 2018. Feb;16(2):121–131. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources