Endothelial TRIM35-Regulated MMP10 Release Exacerbates Calcification of Vascular Grafts
- PMID: 39865905
- PMCID: PMC11923891
- DOI: 10.1002/advs.202409641
Endothelial TRIM35-Regulated MMP10 Release Exacerbates Calcification of Vascular Grafts
Abstract
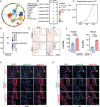
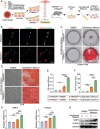
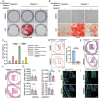
Vascular calcification is a highly regulated process in cardiovascular disease (CVD) and is strongly correlated with morbidity and mortality, especially in the adverse stage of vascular remodeling after coronary artery bypass graft surgery (CABG). However, the pathogenesis of vascular graft calcification, particularly the role of endothelial-smooth muscle cell interaction, is still unclear. To test how ECs interact with SMCs in artery grafts, single-cell analysis of wild-type mice is first performed using an arterial isograft mouse model and found robust cytokine-mediated signaling pathway activation and SMC proliferation, together with upregulated endothelial tripartite motif 35 (TRIM35) expression. Unexpectedly, severe SMC calcification in artery grafts is found in TRIM35 conditional endothelial knockout (cKO) mice. Calcified medium (comprising calcium chloride and beta-glycerophosphate)-induced calcium deposition in vitro is also found in SMCs cocultured with TRIM35 knockout endothelium. This extraordinary phenomenon is further confirmed to be induced by increased MMP10 secretion. Mechanistically, endothelial TRIM35 inhibits MMP10 expression and secretion by promoting K63-linked ubiquitination of RelB and maintaining its nuclear localization, consequently inhibiting nuclear transcription of MMP10 through the noncanonical NF-κB signaling pathway. Targeting MMP10 in situ in arterial isografts can effectively alleviate vascular calcification caused by conditional endothelial TRIM35 knockout. These findings demonstrated that TRIM35 inhibited vascular calcification during arterial isograft remodeling, a process that is driven by the aberrant secretion of endothelial MMP10. Targeting MMP10 pathway may be a potential therapeutic strategy for vascular calcification in vessel grafts.
Keywords: MMP10; TRIM35; calcification; endothelial cells; smooth muscle cells.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Endothelial cells derived extracellular vesicles promote diabetic arterial calcification via circ_0008362/miR-1251-5p/Runx2 axial.Cardiovasc Diabetol. 2024 Oct 17;23(1):369. doi: 10.1186/s12933-024-02440-7. Cardiovasc Diabetol. 2024. PMID: 39420345 Free PMC article.
-
Matrix metalloproteinase-10 deficiency delays atherosclerosis progression and plaque calcification.Atherosclerosis. 2018 Nov;278:124-134. doi: 10.1016/j.atherosclerosis.2018.09.022. Epub 2018 Sep 19. Atherosclerosis. 2018. PMID: 30268068
-
Carbonylation of Runx2 at K176 by 4-Hydroxynonenal Accelerates Vascular Calcification.Circulation. 2024 May 28;149(22):1752-1769. doi: 10.1161/CIRCULATIONAHA.123.065830. Epub 2024 Feb 13. Circulation. 2024. PMID: 38348663
-
Deletion of IκB-Kinase β in Smooth Muscle Cells Induces Vascular Calcification Through β-Catenin-Runt-Related Transcription Factor 2 Signaling.J Am Heart Assoc. 2018 Jan 4;7(1):e007405. doi: 10.1161/JAHA.117.007405. J Am Heart Assoc. 2018. PMID: 29301759 Free PMC article.
-
Orphan Nuclear Receptor NR4A3 Promotes Vascular Calcification via Histone Lactylation.Circ Res. 2024 May 24;134(11):1427-1447. doi: 10.1161/CIRCRESAHA.123.323699. Epub 2024 Apr 17. Circ Res. 2024. PMID: 38629274
References
-
- Mohr F., Morice M., Kappetein A., Feldman T., Ståhle E., Colombo A., Mack M., Holmes D., Morel M., Van Dyck N., Houle V., Dawkins K., Serruys P., Lancet. 2013, 381, 629. - PubMed
-
- Aldea G., Bakaeen F., Pal J., Fremes S., Head S., Sabik J., Rosengart T., Kappetein A., Thourani V., Firestone S., Mitchell J., Ann. Thoracic Surge. 2016, 101, 801. - PubMed
-
- Gaudino M., Benedetto U., Fremes S., Ballman K., Biondi‐Zoccai G., Sedrakyan A., Nasso G., Raman J., Buxton B., Hayward P., Moat N., Collins P., Webb C., Peric M., Petrovic I., Yoo K., Hameed I., Di Franco A., Moscarelli M., Speziale G., Puskas J., Girardi L., Hare D., Taggart D., JAMA, J. Am. Med. Assoc. 2020, 324, 179. - PMC - PubMed
-
- Pober J., Sessa W., Nat. Rev. Immunol. 2007, 7, 803. - PubMed
-
- Mitchell R., Libby P., Circ. Res. 2007, 100, 967. - PubMed
MeSH terms
Substances
Grants and funding
- 82470445/National Natural Science Foundation of China
- 82170437/National Natural Science Foundation of China
- 82030008/National Natural Science Foundation of China
- 32330046/National Natural Science Foundation of China
- 2022YFC3602400/Key Technologies Research and Development Program
- 2022YFC3602401/Key Technologies Research and Development Program
- 2024JJ8118/Regional Joint Fund Program of the Natural Science Foundation of Hunan Province
- 2023CXQD007/Innovation-Driven Project of Central South University
- 2024M753684/China Postdoctoral Science Foundation
- GZB20240870/Postdoctoral Fellowship Program (Grand B) of China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
