DNA Damage Repair in Glioblastoma: A Novel Approach to Combat Drug Resistance
- PMID: 39866010
- PMCID: PMC12179567
- DOI: 10.1111/cpr.13815
DNA Damage Repair in Glioblastoma: A Novel Approach to Combat Drug Resistance
Abstract
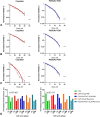
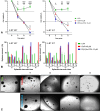
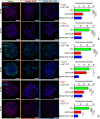
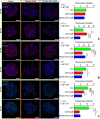
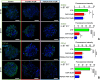
Due to the lack of effective therapeutic approach, glioblastoma (GBM) remains one of the most malignant brain tumour. By in vitro investigations on primary GBM stem cells, we highlighted one of the underlying mechanisms of drug resistance to alkylating agents, the DNA damage responses. Here, flow cytometric analysis and viability and repopulation assays were used to assess the long-term cytotoxic effect induced by the administration of a fourth-generation platinum prodrug, the (OC-6-44)-acetatodiamminedichlorido(2-(2-propynyl)octanoato) platinum(IV) named Pt(IV)Ac-POA, in comparison to the most widely used Cisplatin. The immunofluorescence studies revealed changing pathways involved in the DNA damage response mechanisms in response to the two chemotherapies, suggesting in particular the role of Poly (ADP-Ribose) polymerases in the onset of resistance to Cisplatin-induced cytotoxicity. Thus, this research provides a proof of concept for how the use of a prodrug which allows the co-administration of Cisplatin and an Histone DeACetylase inhibitors, could suppress DNA repair mechanisms, suggesting a novel effective approach in GBM treatment.
Keywords: DNA damage response; chemotherapy; drug resistance; glioblastoma.
© 2025 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

