Negative gas adsorption transitions and pressure amplification phenomena in porous frameworks
- PMID: 39866063
- PMCID: PMC11770586
- DOI: 10.1039/d4cs00555d
Negative gas adsorption transitions and pressure amplification phenomena in porous frameworks
Abstract
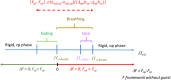
Nanoporous solids offer a wide range of functionalities for industrial, environmental, and energy applications. However, only a limited number of porous materials are responsive, i.e. the nanopore dynamically alters its size and shape in response to external stimuli such as temperature, pressure, light or the presence of specific molecular stimuli adsorbed inside the voids deforming the framework. Adsorption-induced structural deformation of porous solids can result in unique counterintuitive phenomena. Negative gas adsorption (NGA) is such a phenomenon which describes the spontaneous release of gas from an "overloaded" nanoporous solid via adsorption-induced structural contraction leading to total pressure amplification (PA) in a closed system. Such pressure amplifying materials may open new avenues for pneumatic system engineering, robotics, damping, or micromechanical actuators. In this review we illustrate the discovery of NGA in DUT-49, a mesoporous metal-organic framework (MOF), and the subsequent examination of conditions for its observation leading to a rationalization of the phenomenon. We outline the development of decisive experimental and theoretical methods required to establish the mechanism of NGA and derive key criteria for observing NGA in other porous solids. We demonstrate the application of these design principles in a series of DUT-49-related model compounds of which several also exhibit NGA. Furthermore, we provide an outlook towards applying NGA as a pressure amplification material and discuss possibilities to discover novel NGA materials and other counterintuitive adsorption phenomena in porous solids in the future.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
-
- Lin J.-B. Nguyen T. T. T. Vaidhyanathan R. Burner J. Taylor J. M. Durekova H. Akhtar F. Mah R. K. Ghaffari-Nik O. Marx S. Fylstra N. Iremonger S. S. Dawson K. W. Sarkar P. Hovington P. Rajendran A. Woo T. K. Shimizu G. K. H. Science. 2021;374:1464–1469. doi: 10.1126/science.abi7281. - DOI - PubMed
- Shekhah O. Belmabkhout Y. Chen Z. Guillerm V. Cairns A. Adil K. Eddaoudi M. Nat. Commun. 2014;5:4228. doi: 10.1038/ncomms5228. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

