Expression of spider silk protein in tobacco improves drought tolerance with minimal effects on its mechanotype
- PMID: 39866095
- PMCID: PMC11771620
- DOI: 10.1111/tpj.17213
Expression of spider silk protein in tobacco improves drought tolerance with minimal effects on its mechanotype
Abstract
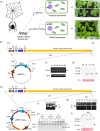
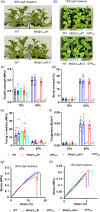
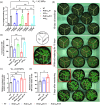
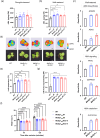
Spider silk, especially dragline silk from golden silk spiders (Trichonephila clavipes), is an excellent natural material with remarkable mechanical properties. Many studies have focused on the use of plants as biofactories for the production of recombinant spider silk. However, the effects of this material on the mechanical properties or physiology of transgenic plants remain poorly understood. Since glycine-rich proteins play key roles in plants, we evaluated the effects of a glycine-rich spider silk protein on plant mechanical properties (mechanotype) and physiology. We generated tobacco (Nicotiana tabacum) plants producing a nucleus- or plastid-encoded partial component of dragline silk, MaSp1 (major ampullate spidroin-1; MaSp1-tobacco), containing six repetitive glycine-rich and polyalanine tandem domains. MaSp1 accumulation had minimal effect on leaf mechanical properties, but improved drought tolerance. Transcriptome analysis of drought-stressed MaSp1-tobacco revealed the upregulation of genes involved in stress response, antioxidant activity, cellular metabolism and homeostasis, and phenylpropanoid biosynthesis. The effects of drought treatment differed between the nucleus- and the plastid-encoded MaSp1-tobacco, with the latter showing a stronger transcriptomic response and a higher total antioxidant status (TAS). Well-watered MaSp1-tobacco displayed elevated levels of the stress phytohormone ABA, leading to stomatal closure, reduced water loss, activation of stress response, and increased TAS. We show that the moderately enhanced ABA content in these plants plays a pivotal role in drought tolerance, alongside, ABA priming, which causes overall adjustments in multiple drought tolerance mechanisms. Thus, our findings highlight the potential of utilizing glycine-rich spider silk proteins to enhance plant resilience to drought.
Keywords: ABA; drought tolerance; glycine‐rich; mechanotype; spider silk; tensile strength.
© 2024 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Low-Tech, Pilot Scale Purification of a Recombinant Spider Silk Protein Analog from Tobacco Leaves.Int J Mol Sci. 2016 Oct 9;17(10):1687. doi: 10.3390/ijms17101687. Int J Mol Sci. 2016. PMID: 27735843 Free PMC article.
-
Multicomponent nature underlies the extraordinary mechanical properties of spider dragline silk.Proc Natl Acad Sci U S A. 2021 Aug 3;118(31):e2107065118. doi: 10.1073/pnas.2107065118. Proc Natl Acad Sci U S A. 2021. PMID: 34312234 Free PMC article.
-
Recombinant production of spider silk protein in Physcomitrella photobioreactors.Plant Cell Rep. 2025 Apr 26;44(5):103. doi: 10.1007/s00299-025-03485-y. Plant Cell Rep. 2025. PMID: 40287554 Free PMC article.
-
Complexity of Spider Dragline Silk.Biomacromolecules. 2022 May 9;23(5):1827-1840. doi: 10.1021/acs.biomac.1c01682. Epub 2022 Apr 4. Biomacromolecules. 2022. PMID: 35378031 Free PMC article. Review.
-
[Primary Structure Characterization and Biosynthesis of Spider Silk Proteins for Multifunctional Biomaterials].Sheng Wu Gong Cheng Xue Bao. 2024 Mar 25;40(3):687-704. doi: 10.13345/j.cjb.230444. Sheng Wu Gong Cheng Xue Bao. 2024. PMID: 38545971 Review. Chinese.
References
-
- Aroca, R. (Ed.). (2012) Plant Responses to Drought Stress: From Morphological to Molecular Features. Berlin, Heidelberg: Springer. Available from: 10.1007/978-3-642-32653-0 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

