Investigating the potential of diffusion tensor atlases to generate anisotropic clinical tumor volumes in glioblastoma patients
- PMID: 39866246
- PMCID: PMC11758580
- DOI: 10.1016/j.phro.2024.100688
Investigating the potential of diffusion tensor atlases to generate anisotropic clinical tumor volumes in glioblastoma patients
Abstract
Background and purpose: Diffusion tensor imaging (DTI) has been proposed to guide the anisotropic expansion from gross tumor volume to clinical target volume (CTV), aiming to integrate known tumor spread patterns into the CTV. This study investigate the potential of using a DTI atlas as an alternative to patient-specific DTI for generating anisotropic CTVs.
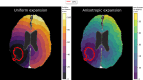
Materials and methods: The dataset consisted of twenty-eight newly diagnosed glioblastoma patients from a Danish national DTI protocol with post-operative T1-contrast and DTI imaging. Three different DTI atlases, spatially aligned to the patient images using deformable image registration, were considered as alternatives. Anisotropic CTVs were constructed to match the volume of a 15 mm isotropic expansion by generating 3D distance maps using either patient- or atlas-DTI as input to the shortest path solver. The degree of CTV anisotropy was controlled by the migration ratio, modeling tumor cell migration along the dominant white matter fiber direction extracted from DTI. The similarity between patient- and atlas-DTI CTVs was analyzed using the Dice Similarity Coefficient (DSC), with significance testing according to a Wilcoxon test.
Results: The median (range) DSC between anisotropic CTVs generated using patient-specific and atlas-based DTI was 0.96 (0.93-0.97), 0.96 (0.93-0.97), and 0.95 (0.93-0.97) for the three atlases, respectively (p 0.01), for a migration ratio of 10. The results remained consistent over the range of studied migration ratios (2 to 100).
Conclusion: The high degree of similarity between all anisotropic CTVs indicates that atlas-DTI is a viable replacement for patient-specific DTI for incorporating fiber direction into the CTV.
Keywords: Anisotropic margin expansion; CTV; DTI; Glioblastoma; Radiotherapy.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Diffusion tensor imaging for target volume definition in glioblastoma multiforme.Strahlenther Onkol. 2014 Oct;190(10):939-43. doi: 10.1007/s00066-014-0676-3. Epub 2014 May 14. Strahlenther Onkol. 2014. PMID: 24823897 Clinical Trial.
-
Diffusion tensor magnetic resonance imaging driven growth modeling for radiotherapy target definition in glioblastoma.Acta Oncol. 2017 Nov;56(11):1639-1643. doi: 10.1080/0284186X.2017.1374559. Epub 2017 Sep 12. Acta Oncol. 2017. PMID: 28893125
-
A deep learning-based framework for segmenting invisible clinical target volumes with estimated uncertainties for post-operative prostate cancer radiotherapy.Med Image Anal. 2021 Aug;72:102101. doi: 10.1016/j.media.2021.102101. Epub 2021 May 17. Med Image Anal. 2021. PMID: 34111573
-
Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group.Acta Oncol. 2013 May;52(4):703-10. doi: 10.3109/0284186X.2013.765064. Epub 2013 Feb 19. Acta Oncol. 2013. PMID: 23421926
-
Diffusion tensor imaging: a review for pediatric researchers and clinicians.J Dev Behav Pediatr. 2010 May;31(4):346-56. doi: 10.1097/DBP.0b013e3181dcaa8b. J Dev Behav Pediatr. 2010. PMID: 20453582 Free PMC article. Review.
Cited by
-
Recent innovations in offline and online Magnetic Resonance Imaging guided radiation oncology.Phys Imaging Radiat Oncol. 2025 Mar 19;34:100753. doi: 10.1016/j.phro.2025.100753. eCollection 2025 Apr. Phys Imaging Radiat Oncol. 2025. PMID: 40213032 Free PMC article. No abstract available.
References
-
- Halperin E.C., Bentel G., Heinz E., Burger P.C. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: An analysis based on post mortem topographic anatomy with ct correlations. Int J Radiat Oncol*Biol*Phys. 1989;17(6):1347–1350. doi: 10.1016/0360-3016(89)90548-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources

