Automated Quantification of Retinopathy of Prematurity Stage via Ultrawidefield OCT
- PMID: 39866345
- PMCID: PMC11760822
- DOI: 10.1016/j.xops.2024.100663
Automated Quantification of Retinopathy of Prematurity Stage via Ultrawidefield OCT
Abstract
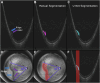
Purpose: Retinopathy of prematurity (ROP) stage is defined by the visual appearance of the vascular-avascular border, which reflects a spectrum of pathologic neurovascular tissue (NVT). Previous work demonstrated that the thickness of the ridge lesion, measured using OCT, corresponds to higher clinical diagnosis of stage. This study evaluates whether the volume of anomalous NVT (ANVTV), defined as abnormal tissue protruding from the regular contour of the retina, can be measured automatically using deep learning to develop quantitative OCT-based biomarkers in ROP.
Design: Single-center retrospective case series.
Participants: Thirty-three infants with ROP in the Oregon Health & Science University neonatal intensive care unit.
Methods: OCT B-scans were collected using an investigational ultrawidefield OCT. The ANVTV was manually segmented. A set of 3347 B-scans and corresponding manual segmentations from 12 volumes from 6 patients were used to train an automated segmentation tool using a U-Net. An additional held-out test data set of 60 B-scans from 6 infants was used to evaluate model performance. The Dice-Sorensen coefficient (DSC) comparing manual and automated segmentation of ANVTV was calculated. Scans from 21 additional infants were used for clinical evaluation of ANVTV using the visit in which they had developed their peak stage of ROP. Each infant had every B-scan in a volume automatically segmented for ANVTV (total number of segmented voxels within the 60° temporal to the optic disc). The ANVTV was compared between infants with stage 1 to 3 ROP using a Kruskal-Wallis test and tracked over time in all infants with stage 3 ROP.
Main outcome measurements: Cross sectional and longitudinal association between ANVTV and stages 1 to 3 ROP.
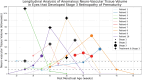
Results: Comparing automated and manual segmentation of ANVTV achieved a DSC of 0.61 ± 0.13. Using the U-Net, ANVTV was associated with higher disease stage both cross sectionally and longitudinally. Median ANVTV significantly increased as ROP stage worsened from 1 (0, [interquartile range: 0-0] kilovoxels) to 2 (170.1 [interquartile range: 104.2-183.6] kilovoxels) to 3 (421.4 [interquartile range: 312.3-1110.8] kilovoxels; P < 0.001).
Conclusions: Automated OCT-based measurement of ANVTV was associated with clinical disease stage in ROP, both cross sectionally and longitudinally. Ultrawidefield-OCT may facilitate more objective screening, diagnosis, and monitoring in the future.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Anomalous neurovascular tissue; Isolated retinal neovascularization; Optical coherence tomography; Retinopathy of prematurity; Ridge.
© 2024 by the American Academy of Ophthalmologyé.
Figures


References
-
- Gschließer A., Stifter E., Neumayer T., et al. Inter-expert and intra-expert agreement on the diagnosis and treatment of retinopathy of prematurity. Am J Ophthalmol. 2015;160:553–560.e3. - PubMed
-
- Foos R.Y. Retinopathy of prematurity. Pathologic correlation of clinical stages. Retina. 1987;7:260–276. - PubMed
-
- Sun Y., Dalal R., Gariano R.F. Cellular composition of the ridge in retinopathy of prematurity. Arch Ophthalmol. 2010;128:638–641. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

