Cancer type-specific adverse events of immune checkpoint inhibitors: A systematic review and meta-analysis
- PMID: 39866435
- PMCID: PMC11757769
- DOI: 10.1016/j.heliyon.2024.e41597
Cancer type-specific adverse events of immune checkpoint inhibitors: A systematic review and meta-analysis
Abstract
Background: The distribution of adverse events (AEs) triggered by immune checkpoint inhibitors (ICIs) across different cancer types has never been demonstrated.
Methods: Randomised controlled trials exclusively assessing ICI monotherapy in cohorts of over 100 patients were considered. Our primary outcome was a comprehensive summary of the distribution of all-grade treatment-related adverse events (TRAEs) as well as serious TRAEs (CTCAE grade 3 or higher) across different malignancies. The study is registered with PROSPERO CRD42023387934.
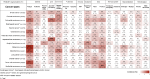
Findings: 75 trials that enrolled over 100 patients were included. While investigating the incidence of each TRAE across various cancers, we found special linkages existed between certain TRAEs and particular cancer types. In anti-PD-1 monotherapy group, melanoma patients experienced the most frequent fatigue (31.1 %, 95 % CI 29.7%-32.5 %); the incidences of severe pneumonitis and other respiratory disorders were highest in Hodgkin lymphoma (4.1 %, 95 % CI 1.5%-8.6 %; 4.1 %, 95 % CI 1.5%-8.6 %, respectively). Among individuals undergoing single-agent anti-PD-L1, higher frequency of all-grade pruritus occurred in 19.0 % of renal cell carcinoma (RCC) patients (95 % CI 15.2%-23.2 %), and the highest probability of developing other severe musculoskeletal disorders was observed in patients with RCC (6.2 %, 95 % CI 4.0%-9.0 %). In anti-CTLA-4 monotherapy, the incidences of both all-grade and severe diarrhea occurred most frequently in prostate cancer patients (41.9 %, 95 % CI 37.9%-47.9; 14.8 %, 95 % CI 11.5%-18.7 %, respectively).
Interpretation: This is the first comprehensive study addressing the distribution of various TRAEs across cancer types. Our research emphasizes the significance of considering cancer-specific TRAEs when using ICIs for treatment.
© 2025 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this manuscript, “Cancer type-specific adverse events of immune checkpoint inhibitors: A systematic review and meta-analysis.” The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 2021;16:223–249. - PubMed
-
- Hussaini S., Chehade R., Boldt R.G., Raphael J., Blanchette P., Maleki Vareki S., et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - a systematic review and meta-analysis. Cancer Treat Rev. 2021;92 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

