Humoral and cellular response to SARS-CoV-2 mRNA vaccine in paediatric heart transplant recipients
- PMID: 39866443
- PMCID: PMC11758410
- DOI: 10.1016/j.heliyon.2024.e41584
Humoral and cellular response to SARS-CoV-2 mRNA vaccine in paediatric heart transplant recipients
Abstract
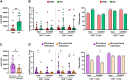
Objective: The aim of this prospective cohort study is to analyse the humoral and cellular vaccine responses in paediatric heart transplant recipients (HTR, n = 12), and compare it with the response in healthy controls (HC, n = 14). All participants were 5-18 years old and vaccinated with mRNA vaccine against SARS-CoV-2 between December 2021 and May 2022.
Methods: The humoral response was measured by quantifying antibody titers against SARS-CoV-2 spike protein (anti-S). The T-lymphocyte phenotype and SARS-CoV2-specific CD4+ and CD8+ T-cell response was studied by multiparametric flow cytometry through peripheral blood mononuclear cells by the quantification of degranulation markers (CD107a) and intracellular cytokines (IFN-γ, TNF-α and IL-2) after in vitro stimulation with SARS-CoV-2 peptides from structural proteins (S, M, N, E) and non-structural viral proteins.
Results: After vaccination, humoral response was found in all HTR, although they showed lower levels of anti-S IgG compared to HC (p = 0.003). However, in terms of cellular response, no significant differences were obtained in the prevalence of responders and magnitude of responses between groups. In addition, anti-S IgG levels directly correlated with a higher SARS-CoV-2 specific T-cell response (rho = 0.43; p = 0.027 and rho = 0.45; p = 0.02 for IFN-γ+ and TNF-α+ production of CD8+ T-cells, respectively). Activated T-cell phenotype in HTR was associated with a lower humoral response to SARS-CoV-2 vaccine.
Conclusion: HTR had humoral response after vaccination, although they showed lower levels of specific anti-S antibodies compared to HC. There were no significant differences in the SARS-CoV2-specific cellular response between the two groups. Obtaining satisfactory data on this type of response could potentially challenge the current vaccine guideline recommendations.
Keywords: COVID-19; Cell-mediated immunity; Heart transplant recipients; Humoral immunity; Immunocompromised; Paediatric; SARS-CoV-2; Vaccines.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Velleca A., Shullo M.A., Dhital K., Azeka E., Colvin M., DePasquale E., Farrero M., García-Guereta L., Jamero G., Khush K., Lavee J., Pouch S., Patel J., Michaud C., Shullo M.A., Schubert S., Angelini A., Carlos L., Mirabet S., Patel J., Pham M., Urschel S., Kim K.-H., Miyamoto S., Chih S., Daly K., Grossi P., Jennings D.L., Kim I., Lim H.S., Miller T., Potena L., Velleca A., Eisen H., Bellumkonda L., Danziger-Isakov L., Dobbels F., Harkess M., Kim D., Lyster H., Peled Y., Reinhardt Z. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023;42:e1–e141. doi: 10.1016/j.healun.2022.10.015. - DOI - PubMed
-
- Chaudhry Z.S., Williams J.D., Vahia A., Fadel R., Parraga Acosta T., Prashar R., Shrivastava P., Khoury N., Pinto Corrales J., Williams C., Nagai S., Abouljoud M., Samaniego-Picota M., Abreu-Lanfranco O., Del Busto R., Ramesh M.S., Patel A., Alangaden G.J. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am. J. Transplant. 2020;20:3051–3060. doi: 10.1111/ajt.16188. - DOI - PMC - PubMed
-
- Tschopp J., L'Huillier A.G., Mombelli M., Mueller N.J., Khanna N., Garzoni C., Meloni D., Papadimitriou-Olivgeris M., Neofytos D., Hirsch H.H., Schuurmans M.M., Müller T., Berney T., Steiger J., Pascual M., Manuel O., Van Delden C. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am. J. Transplant. 2020;20:2876–2882. doi: 10.1111/ajt.16062. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

