Biomaterial-assisted organoid technology for disease modeling and drug screening
- PMID: 39866785
- PMCID: PMC11757232
- DOI: 10.1016/j.mtbio.2024.101438
Biomaterial-assisted organoid technology for disease modeling and drug screening
Abstract
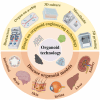
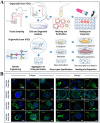
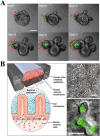
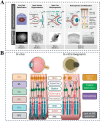
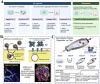
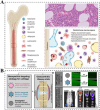
Developing disease models and screening for effective drugs are key areas of modern medical research. Traditional methodologies frequently fall short in precisely replicating the intricate architecture of bodily tissues and organs. Nevertheless, recent advancements in biomaterial-assisted organoid technology have ushered in a paradigm shift in biomedical research. This innovative approach enables the cultivation of three-dimensional cellular structures in vitro that closely emulate the structural and functional attributes of organs, offering physiologically superior models compared to conventional techniques. The evolution of biomaterials plays a pivotal role in supporting the culture and development of organ tissues, thereby facilitating more accurate disease state modeling and the rigorous evaluation of drug efficacy and safety profiles. In this review, we will explore the roles that various biomaterials play in organoid development, examine the fundamental principles and advantages of utilizing these technologies in constructing disease models, and highlight recent advances and practical applications in drug screening using disease-specific organoid models. Additionally, the challenges and future directions of organoid technology are discussed. Through continued research and innovation, we aim to make remarkable strides in disease treatment and drug development, ultimately enhancing patient quality of life.
Keywords: Biomaterial; Disease modeling; Drug screening; Organoid technology.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology.Cells Tissues Organs. 2025;214(2):128-147. doi: 10.1159/000541416. Epub 2024 Sep 12. Cells Tissues Organs. 2025. PMID: 39265553 Free PMC article. Review.
-
Advances of Cell Printing Technology in Organoid Engineering.Tissue Eng Part B Rev. 2025 Jun 12. doi: 10.1089/ten.teb.2025.0048. Online ahead of print. Tissue Eng Part B Rev. 2025. PMID: 40501290 Review.
-
iPSC-derived and Patient-Derived Organoids: Applications and challenges in scalability and reproducibility as pre-clinical models.Curr Res Toxicol. 2024 Oct 2;7:100197. doi: 10.1016/j.crtox.2024.100197. eCollection 2024. Curr Res Toxicol. 2024. PMID: 40276485 Free PMC article.
-
Technological advances and challenges in constructing complex gut organoid systems.Front Cell Dev Biol. 2024 Aug 14;12:1432744. doi: 10.3389/fcell.2024.1432744. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39206092 Free PMC article. Review.
-
Advancing cancer research through organoid technology.J Transl Med. 2024 Nov 8;22(1):1007. doi: 10.1186/s12967-024-05824-1. J Transl Med. 2024. PMID: 39516934 Free PMC article. Review.
Cited by
-
Rational design matrix materials for organoid development and application in biomedicine.Regen Biomater. 2025 May 14;12:rbaf038. doi: 10.1093/rb/rbaf038. eCollection 2025. Regen Biomater. 2025. PMID: 40556786 Free PMC article. Review.
-
From niche to organoid: Engineering bone tissues through microenvironmental insights.J Tissue Eng. 2025 Jul 29;16:20417314251358567. doi: 10.1177/20417314251358567. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 40755459 Free PMC article. Review.
-
Extracellular Matrix Stiffness: Mechanotransduction and Mechanobiological Response-Driven Strategies for Biomedical Applications Targeting Fibroblast Inflammation.Polymers (Basel). 2025 Mar 20;17(6):822. doi: 10.3390/polym17060822. Polymers (Basel). 2025. PMID: 40292716 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

