Oxygenation and function of endocrine bioartificial pancreatic tissue constructs under flow for preclinical optimization
- PMID: 39866963
- PMCID: PMC11758540
- DOI: 10.1177/20417314241284826
Oxygenation and function of endocrine bioartificial pancreatic tissue constructs under flow for preclinical optimization
Abstract
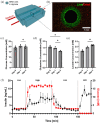
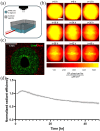
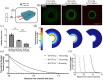
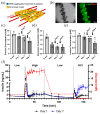
Islet transplantation and more recently stem cell-derived islets were shown to successfully re-establish glycemic control in people with type 1 diabetes under immunosuppression. These results were achieved through intraportal infusion which leads to early graft losses and limits the capacity to contain and retrieve implanted cells in case of adverse events. Extra-hepatic sites and encapsulation devices have been developed to address these challenges and potentially create an immunoprotective or immune-privileged environment. Many strategies have achieved reversal of hyperglycemia in diabetic rodents. So far, the results have been less promising when transitioning to humans and larger animal models due to challenges in oxygenation and insulin delivery. We propose a versatile in vitro perfusion system to culture and experimentally study the function of centimeter-scale tissues and devices for insulin-secreting cell delivery. The system accommodates various tissue geometries, experimental readouts, and oxygenation tensions reflective of potential transplantation sites. We highlight the system's applications by using case studies to explore three prominent bioartificial endocrine pancreas (BAP) configurations: (I) with internal flow, (II) with internal flow and microvascularized, and (III) without internal flow. Oxygen concentration profiles modeled computationally were analogous to viability gradients observed experimentally through live/dead endpoint measurements and in case I, time-lapse fluorescence imaging was used to monitor the viability of GFP-expressing cells in real time. Intervascular BAPs were cultured under flow for up to 3 days and BAPs without internal flow for up to 7 days, showing glucose-responsive insulin secretion quantified through at-line non-disruptive sampling. This system can complement other preclinical platforms to de-risk and optimize BAPs and other artificial tissue designs prior to clinical studies.
Keywords: Bioreactor; diabetes; oxygenation; perfusion; vascularization.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
In vitro oxygen imaging of acellular and cell-loaded beta cell replacement devices.Sci Rep. 2023 Sep 20;13(1):15641. doi: 10.1038/s41598-023-42099-w. Sci Rep. 2023. PMID: 37730815 Free PMC article.
-
A newly developed immunoisolated bioartificial pancreas with cell sheet engineering.Cell Transplant. 2008;17(1-2):51-9. doi: 10.3727/000000008783907035. Cell Transplant. 2008. PMID: 18468235
-
Design of Bioartificial Pancreases From the Standpoint of Oxygen Supply.Artif Organs. 2018 Aug;42(8):E168-E185. doi: 10.1111/aor.13106. Epub 2018 Apr 2. Artif Organs. 2018. PMID: 29611212
-
Oxygenation strategies for encapsulated islet and beta cell transplants.Adv Drug Deliv Rev. 2019 Jan 15;139:139-156. doi: 10.1016/j.addr.2019.05.002. Epub 2019 May 8. Adv Drug Deliv Rev. 2019. PMID: 31077781 Review.
-
Progress and challenges of the bioartificial pancreas.Nano Converg. 2016;3(1):28. doi: 10.1186/s40580-016-0088-4. Epub 2016 Nov 1. Nano Converg. 2016. PMID: 28191438 Free PMC article. Review.
Cited by
-
Assessing Implantation Sites for Pancreatic Islet Cell Transplantation: Implications for Type 1 Diabetes Mellitus Treatment.Bioengineering (Basel). 2025 May 9;12(5):499. doi: 10.3390/bioengineering12050499. Bioengineering (Basel). 2025. PMID: 40428118 Free PMC article. Review.
References
-
- Gregory GA, Robinson TI, Linklater SE, et al.. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 2022; 10: 741-760. - PubMed
-
- Latres E, Finan DA, Greenstein JL, et al.. Navigating two roads to glucose normalization in diabetes: automated insulin delivery devices and cell therapy. Cell Metab 2019; 29: 545–563. - PubMed
-
- Lablanche S, Vantyghem M-C, Kessler L, et al.. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2018; 6: 527–537. - PubMed
LinkOut - more resources
Full Text Sources

