Cutaneous Manifestations of Systemic Lupus Erythematosus and Their Correlation With Cardiac Involvement
- PMID: 39866976
- PMCID: PMC11769098
- DOI: 10.7759/cureus.76478
Cutaneous Manifestations of Systemic Lupus Erythematosus and Their Correlation With Cardiac Involvement
Abstract
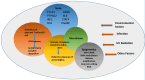
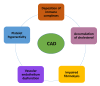
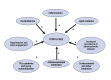
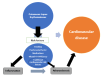
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by widespread immune dysregulation that affects multiple organ systems, including the skin and cardiovascular system. The crosstalk between different cell death pathways-such as apoptosis, necroptosis, and neutrophil extracellular trap (NETosis), plays a pivotal role in the pathogenesis of SLE, influencing both cutaneous and cardiac manifestations. Cutaneous lupus erythematosus (CLE) is one of the most common early signs of SLE, affecting up to 80% of patients. CLE presents in several forms, including acute, subacute, and chronic lesions, each with varying degrees of association with systemic disease. Cardiac involvement, although often underrecognized, significantly contributes to morbidity and mortality in SLE patients, manifesting as pericarditis, myocarditis, valvular disease, and accelerated atherosclerosis. Emerging research suggests that these cutaneous and cardiac manifestations may be connected through shared immune mechanisms, including immune complex deposition, endothelial dysfunction, and chronic inflammation driven by cytokines such as Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). The severity of skin involvement may correlate with an increased risk of cardiovascular events, underscoring the importance of early diagnosis and a multidisciplinary approach to treatment. This review explores the crosstalk among cell death pathways in SLE and examines how these pathways contribute to both cutaneous and cardiac manifestations. Furthermore, it highlights the clinical implications of this crosstalk and discusses potential therapeutic strategies aimed at modulating these cell death pathways to improve patient outcomes. Challenges and gaps in current research are also addressed, emphasizing the need for further investigation into these complex interactions.
Keywords: autoimmune disease and heart complications; biologics in lupus management; cardiac involvement in sle; cutaneous manifestations; immunosuppressive therapy in sle; lupus skin lesions; multidisciplinary care in sle; skin-cardiac correlation in lupus; systemic lupus erythematosus (sle).
Copyright © 2024, Zeb et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
Pathological mechanisms and crosstalk among various cell death pathways in cardiac involvement of systemic lupus erythematosus.Front Immunol. 2024 Sep 5;15:1452678. doi: 10.3389/fimmu.2024.1452678. eCollection 2024. Front Immunol. 2024. PMID: 39301029 Free PMC article. Review.
-
Exploring cardiovascular implications in systemic lupus erythematosus: A holistic analysis of complications, diagnostic criteria, and therapeutic modalities, encompassing pharmacological and adjuvant approaches.Biomol Concepts. 2024 Nov 27;15(1):10.1515/bmc-2022-0051. doi: 10.1515/bmc-2022-0051. eCollection 2024 Jan 1. Biomol Concepts. 2024. PMID: 39603656 Review.
-
Immunogenetics of cutaneous lupus erythematosus.Curr Opin Pediatr. 2016 Aug;28(4):470-5. doi: 10.1097/MOP.0000000000000383. Curr Opin Pediatr. 2016. PMID: 27386968 Free PMC article. Review.
-
Dermatologic Manifestations, Histologic Features and Disease Progression among Cutaneous Lupus Erythematosus Subtypes: A Prospective Observational Study in Asians.Dermatol Ther (Heidelb). 2021 Feb;11(1):131-147. doi: 10.1007/s13555-020-00471-y. Epub 2020 Dec 5. Dermatol Ther (Heidelb). 2021. PMID: 33280074 Free PMC article.
-
Atypical and Rare Forms of Cutaneous Lupus Erythematosus: The Importance of the Diagnosis for the Best Management of Patients.Dermatology. 2022;238(2):195-204. doi: 10.1159/000515766. Epub 2021 Jun 3. Dermatology. 2022. PMID: 34082424 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
