The protective effect of zinc oxide nanoparticles on boar sperm during preservation at 17 °C
- PMID: 39867304
- PMCID: PMC11758902
- DOI: 10.1590/1984-3143-AR2023-0013
The protective effect of zinc oxide nanoparticles on boar sperm during preservation at 17 °C
Abstract
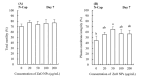
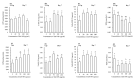
More than 90% of spermatozoa of boars in pork producing countries is stored in liquid at 17 °C; however, the quality of these spermatozoa is affected by bacterial breeding and oxidative damage. This study analyzed sperm quality and sperm capacitation after storage to study the effects of the effects of ZnO nanoparticles (ZnO NPs) supplementation on seminal plasma (SP)-free sperm preservation. We investigated the effects of adding 20, 50, 100 and 200 μg/mL of ZnO NPs to a seminal free boar sperm diluent over a 7-day period at 17 °C to assess the changes in non-capacitated/capacitated sperm quality parameters, antioxidant capacity, ATP content and extent of protein tyrosine phosphorylation. The addition of different doses of ZnO NPs to stored sperm did not induce significant effects on the sperm motility and ATP content when compared to the sperm without ZnO NPs treatment. However, the addition of 50, 100, 200 μg/mL ZnO NPs to stored sperm improved total antioxidant capacity (T-AOC) and CuZn-superoxide dismutase (CuZn-SOD) (p < 0.05). ZnO NPs also reduced the malondialdehyde (MDA) content of the preserved sperm (p < 0.05). Moreover, our results indicate that the supplementation of 50 μg/mL ZnO NPs to preserved sperm improved the sperm membrane integrity (p < 0.05). ZnO NPs exerted protective effects on protein tyrosine phosphorylation, especially with regards to membrane proteins. Following incubation and capacitation, sperm exhibited good levels of protein tyrosine phosphorylation and ATP levels with high T-AOC and CuZn-SOD activity and low MDA content. ZnO NPs exerted protective capacity to a preservation extender used for SP-free boar sperm during storage at 17 °C. The optimal concentration of ZnO NPs for preservation extender was 50 μg/mL.
Keywords: capacitation; oxidative stress; protein; sperm.
Copyright © The Author(s).
Conflict of interest statement
Conflicts of interest: The authors have no conflict of interest to declare.
Figures



Similar articles
-
Effects of zinc chloride on boar sperm quality during liquid storage at 17°C.Vet Med Sci. 2023 May;9(3):1217-1225. doi: 10.1002/vms3.1094. Epub 2023 Feb 9. Vet Med Sci. 2023. PMID: 36757104 Free PMC article.
-
Effects of different concentrations of zinc oxide nanoparticles on the quality of ram cauda epididymal spermatozoa during storage at 4°C.Reprod Domest Anim. 2022 Aug;57(8):864-875. doi: 10.1111/rda.14130. Epub 2022 May 1. Reprod Domest Anim. 2022. PMID: 35467051
-
Synthesis of green zinc-oxide nanoparticles and its dose-dependent beneficial effect on spermatozoa during preservation: sperm functional integrity, fertility and antimicrobial activity.Front Bioeng Biotechnol. 2024 Feb 23;12:1326143. doi: 10.3389/fbioe.2024.1326143. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38464542 Free PMC article.
-
ZnO NPs protect boar sperm in liquid storage through increasing the phosphorylation of PKAs.Anim Reprod. 2025 Apr 18;22(2):e20240025. doi: 10.1590/1984-3143-AR2024-0025. eCollection 2025. Anim Reprod. 2025. PMID: 40276358 Free PMC article.
-
Rosmarinic acid improves boar sperm quality, antioxidant capacity and energy metabolism at 17°C via AMPK activation.Reprod Domest Anim. 2020 Dec;55(12):1714-1724. doi: 10.1111/rda.13828. Epub 2020 Oct 14. Reprod Domest Anim. 2020. PMID: 32969084 Review.
References
-
- Britto FA, Cortade F, Belloum Y, Blaquière M, Gallot YS, Docquier A, Pagano AF, Jublanc E, Bendridi N, Koechlin-Ramonatxo C, Chabi B, Francaux M, Casas F, Freyssenet D, Rieusset J, Giorgetti-Peraldi S, Carnac G, Ollendorff V, Favier FB. Glucocorticoid-dependent REDD1 expression reduces muscle metabolism to enable adaptation under energetic stress. BMC Biol. 2018;16(1):65. doi: 10.1186/s12915-018-0525-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
