Cu/Cu2O/NH2-MIL-88B (Fe) heterojunction as the photocatalyst to remove hexavalent chromium heavy metal ions in water
- PMID: 39867318
- PMCID: PMC11758217
- DOI: 10.1039/d4ra08143a
Cu/Cu2O/NH2-MIL-88B (Fe) heterojunction as the photocatalyst to remove hexavalent chromium heavy metal ions in water
Abstract
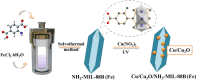
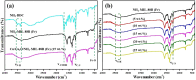
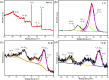
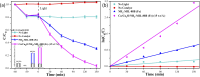
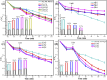
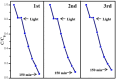
This work aimed at addressing the problem of hexavalent chromium pollution in the water environment, designing and preparing the Cu/Cu2O/NH2-MIL-88B (Fe) heterojunction material with NH2-MIL-88B (Fe) as the carrier, Cu/Cu2O was loaded on NH2-MIL-88B (Fe) by light-assisted reduction. The loading of Cu2O effectively improves the visible light absorption capacity of the composite material. The SPR effect of Cu improves the separation and transfer of photogenerated carriers in the composite material. Performance test results showed that under the condition of pH = 2, using ethanol as a sacrificial agent, Cu/Cu2O/NH2-MIL-88B (Fe) (15 wt%) had a photocatalytic adsorption reduction rate of Cr(vi) of 96.3% in 60 minutes of adsorption in the dark and 150 minutes of photocatalytic reduction, which was 1.39 times that of NH2-MIL-88B (Fe). In addition, the composite material had good stability and recyclability, and the reduction efficiency still reached 88.9% after three cycles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Müller M. Hermes S. Kähler K. van den Berg M. W. E. Muhler M. Fischer R. A. Chem. Mater. 2008;20:4576–4587.
-
- Shi Z. Zhang Y. Duoerkun G. Cao W. Liu T. Zhang L. Liu J. Li M. Chen Z. Environ. Sci.: Nano. 2020;7:2708–2722.
-
- Yang D. Fang W. Zhang H. Sun H. Gu X. Chen H. Luo J. J. Hazard. Mater. 2024;476:134985. - PubMed
-
- Jamaluddin N. S. Alias N. H. Samitsu S. Othman N. H. Jaafar J. Marpani F. Lau W. J. Tan Y. Z. J. Environ. Chem. Eng. 2022;10:108665.
LinkOut - more resources
Full Text Sources

