Research on transition metals for the multicomponent synthesis of benzo-fused γ-lactams
- PMID: 39867320
- PMCID: PMC11756498
- DOI: 10.1039/d4ra08798d
Research on transition metals for the multicomponent synthesis of benzo-fused γ-lactams
Abstract
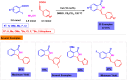
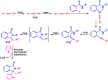
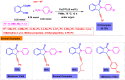
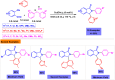
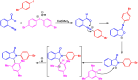
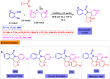
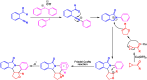
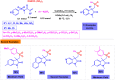
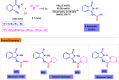
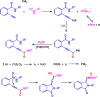
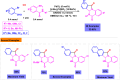
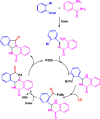
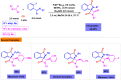
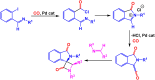
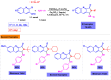
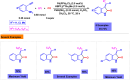
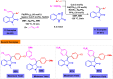
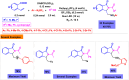
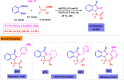
Benzo-fused γ-lactams are fundamental in medicinal chemistry, acting as essential elements for various therapeutic agents due to their structural adaptability and capability to enhance biological activity. In their synthesis, transition metals play a pivotal role as catalysts, offering more efficient alternatives to traditional methods by facilitating C-N bond formation through mechanisms like intramolecular coupling. Recent advances have especially spotlighted transition-metal-catalyzed C-H amination reactions for directly converting C(sp2)-H to C(sp2)-N bonds, streamlining the creation of these compounds. Furthermore, biocatalytic approaches have emerged, providing asymmetric synthesis of lactams with high yield and enantioselectivity. This review examined the transition metal-catalyzed synthesis techniques for producing benzo-fused γ-lactams, marking a significant leap in organic synthesis by proposing more effective, selective, and greener production methods. It serves as a valuable resource for researchers in the fields of transition metal catalysts and those engaged in synthesizing these lactams.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Lalut J. Hocine S. Maertens G. Vilchis-Reyes M. A. Hanessian S. J. Mol. Struct. 2023;14:137104.
-
- Tang Z. Tan Y. Y. Chen H. Wan Y. Curr. Med. Chem. 2022;30:372–389. - PubMed
-
- Pharande S. G. Synthesis. 2021;53:418–446.
-
- Parrino B. Ciancimino C. Carbone A. Spanò V. Montalbano A. Barraja P. Cirrincione G. Diana P. Chem. Inf. 2015;94:149. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

