An Unexpected Water Channel in the Light-Harvesting Complex of a Diatom: Implications for the Switch between Light Harvesting and Photoprotection
- PMID: 39867443
- PMCID: PMC11758497
- DOI: 10.1021/acsphyschemau.4c00069
An Unexpected Water Channel in the Light-Harvesting Complex of a Diatom: Implications for the Switch between Light Harvesting and Photoprotection
Abstract
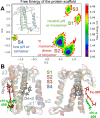
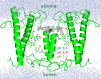
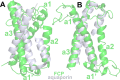
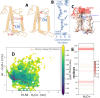
Many important processes in cells depend on the transfer of protons through water wires embedded in transmembrane proteins. Herein, we have performed more than 55 μs all-atom simulations of the light-harvesting complex of a diatom, i.e., the fucoxanthin and chlorophyll a/c binding protein (FCP) from the marine diatom Phaeodactylum tricornutum. Diatoms are unique models to study natural photosynthesis as they exert an efficient light-harvesting machinery with a robust pH-dependent photoprotective mechanism. The present study reports on the dynamics of an FCP monomer, a dimer, and a tetramer at varying pH values. Surprisingly, we have identified at low pH a water channel across FCP that selectively hydrates and protonates the acrylate of a Chl-c2 pigment located in the middle of the membrane. These results are further supported by QM/MM calculations and steered MD simulations on the proton dynamics. It is shown that proton hopping events between the lumenal and stromal sides of the membrane through the observed water channel are highly disfavored. This hindrance is due to the presence of residues Arg31 and Lys82 close to the acrylate, along with an hydronium desolvation penalty that shows close similarities to the water conductance in aquaporins. Furthermore, we provide strong evidence that this identified water channel is governing the transition between light-harvesting and photoprotective states of the major FCP complex in the diatom P. tricornutum.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Conservation of triplet-triplet energy transfer photoprotective pathways in fucoxanthin chlorophyll-binding proteins across algal lineages.Biochim Biophys Acta Bioenerg. 2023 Apr 1;1864(2):148935. doi: 10.1016/j.bbabio.2022.148935. Epub 2022 Nov 13. Biochim Biophys Acta Bioenerg. 2023. PMID: 36379269
-
Adaptation of light-harvesting and energy-transfer processes of a diatom Phaeodactylum tricornutum to different light qualities.Photosynth Res. 2020 Dec;146(1-3):227-234. doi: 10.1007/s11120-020-00714-1. Epub 2020 Jan 21. Photosynth Res. 2020. PMID: 31965467
-
Structural features of the diatom photosystem II-light-harvesting antenna complex.FEBS J. 2020 Jun;287(11):2191-2200. doi: 10.1111/febs.15183. Epub 2020 Jan 7. FEBS J. 2020. PMID: 31854056 Review.
-
Electric Field Susceptibility of Chlorophyll c Leads to Unexpected Excitation Dynamics in the Major Light-Harvesting Complex of Diatoms.J Phys Chem Lett. 2024 Mar 7;15(9):2499-2510. doi: 10.1021/acs.jpclett.3c03241. Epub 2024 Feb 27. J Phys Chem Lett. 2024. PMID: 38410961 Free PMC article.
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein.Photosynth Res. 2023 Apr;156(1):163-177. doi: 10.1007/s11120-022-00935-6. Epub 2022 Jul 10. Photosynth Res. 2023. PMID: 35816266 Free PMC article. Review.
Cited by
-
Synergistic effects of temperature and light on photoprotection in the model diatom Phaeodactylum tricornutum.Physiol Plant. 2025 Jan-Feb;177(1):e70039. doi: 10.1111/ppl.70039. Physiol Plant. 2025. PMID: 39810597 Free PMC article.
References
-
- Kratochvil H. T.; Watkins L. C.; Mravic M.; Thomaston J. L.; Nicoludis J. M.; Somberg N. H.; Liu L.; Hong M.; Voth G. A.; DeGrado W. F. Transient Water Wires Mediate Selective Proton Transport in Designed Channel Proteins. Nat. Chem. 2023, 15 (7), 1012–1021. 10.1038/s41557-023-01210-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
