Effects of combined inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizosphere bacteria on seedling growth and rhizosphere microecology
- PMID: 39867496
- PMCID: PMC11758927
- DOI: 10.3389/fmicb.2024.1475485
Effects of combined inoculation of arbuscular mycorrhizal fungi and plant growth-promoting rhizosphere bacteria on seedling growth and rhizosphere microecology
Abstract
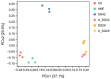
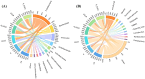
The effects of rhizosphere microorganisms on plant growth and the associated mechanisms are a focus of current research, but the effects of exogenous combined inoculation with arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) on seedling growth and the associated rhizosphere microecological mechanisms have been little reported. In this study, a greenhouse pot experiment was used to study the effects of single or double inoculation with AM fungi (Funneliformis mosseae) and two PGPR (Bacillus sp., Pseudomonas sp.) on the growth of tobacco seedlings, together with high-throughput sequencing technology to reveal associated rhizosphere microecological mechanisms. All inoculation treatments significantly increased the aboveground dry weight; root dry weight; seedling nitrogen, phosphorus, and potassium uptake; plant height; stem thickness; maximum leaf area; chlorophyll content; total root length, surface area, and volume; and average root diameter. The highest values for these indices were observed in the combined treatment of F. mosseae and Pseudomonas sp. SG29 (A_SG29). Furthermore, the A_SG29 treatment yielded the highest diversity indexes and largest percentages of significantly enriched bacterial taxa, and significantly promoted the colonization of AMF in tobacco roots and Pseudomonas in rhizosphere soil. Differential metabolic-pathway predictions using PICRUSt2 showed that the A_SG29 treatment significantly increased the metabolic pathway richness of tobacco rhizosphere microorganisms, and significantly up-regulated some metabolic pathways that may benefit plant growth. Co-inoculation with F. mosseae and Pseudomonas sp. SG29 promoted tobacco-seedling growth by significantly improving rhizosphere microbial communities' structure and function. In summary, the combined inoculation of AMF and SG29 promotes tobacco seedling growth, optimizes the rhizosphere microbial community's structure and function, and serves as a sustainable microbial co-cultivation method for tobacco seedling production.
Keywords: arbuscular mycorrhizal fungi; growth attributes; illumina sequencing; microecological mechanisms; plant growth-promoting rhizobacteria.
Copyright © 2025 Zeng, Xiang, Li, Gao, Chen, Wang, Qian, Wang, Li, Mi, Huang, Xu, Zhao, Zhang and Xiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Angúlo-Castro A., Ferrera-Cerrato R., Alarcón A., Almaraz-Suárez J. J., Delgadillo-Martínez J., Jiménez-Fernández M., et al. . (2021). Improved growth of bell pepper (Capsicum annuum) plants by inoculating arbuscular mycorrhizal fungi and beneficial rhizobacteria. Sci. Fungorum 51:1299. 10.33885/sf.2021.51.1299 - DOI
-
- Anith K. N., Sreekumar A., Sreekumar J. (2015). The growth of tomato seedlings inoculated with co-cultivated Piriformospora indica and Bacillus pumilus. Symbiosis 65, 9–16. 10.1007/s13199-015-0313-7 - DOI
LinkOut - more resources
Full Text Sources

