Pleiotropic effect of teneligliptin versus glimepiride add-on therapy on hs-CRP and cardiorenal parameters in Indian type 2 diabetes patients: An open-labeled randomized controlled trial
- PMID: 39867519
- PMCID: PMC11759232
- DOI: 10.4103/picr.picr_265_23
Pleiotropic effect of teneligliptin versus glimepiride add-on therapy on hs-CRP and cardiorenal parameters in Indian type 2 diabetes patients: An open-labeled randomized controlled trial
Abstract
Objective: The objective of the study was to estimate the pleiotropic effect of teneligliptin on high-sensitivity C-reactive protein (hs-CRP) levels and some cardiorenal parameters in comparison to glimepiride, both as add-on therapy to metformin.
Methodology: This 12-week open-label, parallel-group, randomized controlled trial was conducted among Indian people with type 2 diabetes mellitus and on metformin monotherapy with poor glycemic control (glycated hemoglobin >7% or 53 mmol/mol). The endpoints were mean change in hs-CRP levels, systolic blood pressure (SBP), diastolic blood pressure (DBP), serum creatinine, blood urea, estimated glomerular filtration rate (eGFR), and change in cardiovascular (CV) risk categories from baseline to end of 12 weeks.
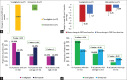
Results: Seventy participants were randomized (1:1) to receive either teneligliptin 20 mg once daily (n = 35) or glimepiride 1 mg twice daily (BD) (n = 35) as an add-on to metformin 500 mg BD. The mean age of the participants was 50.65 and 50.7 years in arms 1 and 2, respectively. At 12-weeks end, teneligliptin add-on caused a statistically significant reduction in hs-CRP compared to glimepiride in both per-protocol (PP) and intention-to-treat (ITT) sets. No significant difference was observed for changes in SBP and DBP, creatinine, urea, eGFR levels, and CV risk category in both PP and ITT sets.
Conclusion: Teneligliptin add-on resulted in favorable effects on hs-CRP levels and comparable effects on cardiorenal parameters compared to glimepiride add-on therapy at 12-weeks end.This trial has been prospectively registered in CTRI (Clinical Trials Registry of India). Registration number: CTRI/2021/08/035342.
Keywords: Dipeptidyl peptidase-4 inhibitors; glimepiride; high-sensitivity C-reactive protein; pleiotropic effects; teneligliptin; type 2 diabetes.
Copyright: © 2024 Perspectives in Clinical Research.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Sapra A, Bhandari P. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [[Last accessed on 2023 Jan 19]]. Diabetes mellitus. Available from: http://www.ncbi.nlm.nih.gov/books/NBK551501/
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract. 2019;157:107843. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
