Ketamine Infusion as an Adjunct to Opioid Analgesia in Pediatric Patients with High-Risk Neuroblastoma Undergoing Treatment with Dinutuximab: Adverse Effects and Safety in a Non-ICU Setting
- PMID: 39867536
- PMCID: PMC11761146
- DOI: 10.2147/JPR.S487724
Ketamine Infusion as an Adjunct to Opioid Analgesia in Pediatric Patients with High-Risk Neuroblastoma Undergoing Treatment with Dinutuximab: Adverse Effects and Safety in a Non-ICU Setting
Abstract
Introduction: Anti-GD2 immunotherapy has improved outcomes for children with high-risk neuroblastoma (HRNBL). Dinutuximab promotes complement-mediated reaction against disialoganglioside GD2, which is expressed in peripheral nerves and over-expressed in neuroblastoma. Dinutuximab is associated with ≥grade 3 neuropathic pain. Targeting GD2 stimulates the NMDA receptor, which makes ketamine useful in treatment of associated pain. The objective of this retrospective study is to describe the use of ketamine for pain uncontrolled by opioids, and ketamine's impact on total opioid usage for patients receiving dinutuximab. In addition, the secondary objective is to describe the toxicities of pain management with opioids versus opioid plus ketamine.
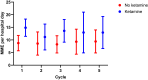
Methods: A retrospective chart review of 40 hRNBL patients receiving dinutuximab at Nationwide Children's Hospital, from 2010 to 2022, was conducted. Demographics, pain scores, medication records, and total daily IV morphine milligram equivalents (IVMME) with and without a ketamine adjunct were collected. Linear mixed effect regression was used to model IVMME use for pain management across dinutuximab cycles and explore the effect of ketamine.
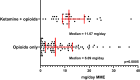
Results: The study cohort included 187 dinutuximab hospitalizations from 40 patients. Age at diagnosis ranged from 1.2 to 11.4 years. 66/187 hospitalizations included ketamine. The average daily IVMME during post-consolidation dinutuximab infusions was greater in admissions with ketamine (median 11.67 mg/day vs 6.09 mg/day; p = 0.0005). Ketamine was not significantly associated (p = 0.77) with daily IVMME when examining opioid use longitudinally over dinutuximab cycles and controlling for patient age. Fever/chills was more frequent in admissions that utilized ketamine (79% vs 63%; p = 0.0297). No other significant statistical differences in adverse effects were observed in patients' receiving opioids versus opioids plus ketamine.
Conclusion: Findings suggest ketamine is safe in a non-ICU setting for treatment of complex pain during anti-GD2 immunotherapy. Additional prospective studies are needed to quantify the effect of reducing opioid side effects by including ketamine in pain management plans.
Keywords: anti-GD2 immunotherapy; dinutuximab; high-risk neuroblastoma; ketamine; neuropathic pain; opioid; pediatric analgesia.
© 2025 Streby et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- WHO definition of Palliative Care. 2020. Available from: https://www.who.int/cancer/palliative/definition/en/. Accessed January 15, 2025.
LinkOut - more resources
Full Text Sources